Introduction
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Chapter:- 1
CHEMICAL REACTIONS & EQUATIONS
Introduction
We come across a variety of changes around us which may be both physical and chemical. A physical change can be easily reversed, but it is not easy to reverse a chemical change. Some common examples of physical change are evaporation, melting of wax, freezing of water, etc and common examples of chemical change are changing of milk to curd, rusting of iron, digestion of food, etc.
All chemical changes are accompanied by chemical reactions and these are represented with the help of chemical equations. In this chapter, we will study about the various types of chemical reactions and the chemical equations which represent chemical changes.
Chemical Reaction
A chemical reaction is a change in which one or more substance(s) or reactant(s) react to form new substance(s) with entirely different properties. Breaking of the old bonds and formation of the new bonds is responsible for the occurrence of a chemical reaction.
Some examples where chemical reactions take place are
(i):- Digestion of food,
(ii):- Rusting of iron,
(iii):- Photosynthesis,
(iv):- Respiration,
(v):- Burning of fuels
A chemical reaction represents the change of the species taking part in the reaction into new species. The reacting species are known as reactants (The substances that undergo chemical change in the chemical reaction, are the reactants) and the new species formed as a result of the reaction are called products (The new substances formed during reaction, are the products).
A chemical reaction can be identified by either of the following observations:-
(i):- Change in state,
(ii):- Change in colour,
(iii):- Evolution of gas↑,
(iv):- Change in temperature,
(v):- Formation of a precipitate↓.
There are some chemical reactions which can show more than one characteristics.
For example, reaction between lead nitrate and potassium iodide solution shows two characteristics; formation of a precipitate of leads iodide and change in colour (from colourless to yellow).
Similarly, reaction between zinc granules and dilute hydrochloric acid shows two characteristics; evolution of a hydrogen gas and change in temperature (rise in temperature).
Chemical Equation
A chemical equation is the symbolic representation of a chemical reaction. Symbols and formulae of the reactants and products are used for the same.
For example, the reaction of burning of methane gas can be represented by the following equation
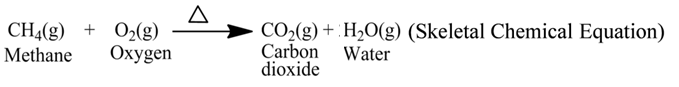
![]()
Making a Chemical Equation More Informative
A chemical equation gives idea about the various substances taking part in a reaction and also about their atoms or molecules included in the reaction. It also tells about the nature of reaction whether it is reversible or not. But following facts remain unexplained with the help of a chemical equation
(i):- Physical states of substances,
(ii):- Reaction conditions,
(iii):- Evolution / absorption of energy
(iv):- Kinetics of the reactions.
Balanced Chemical Equation
A balanced chemical equation is that in which the total number of atoms of each element are equal on both sides of the equation.
Need to Balance a Chemical Equation
According to the law of conservation of mass, 'mass can neither be created nor destroyed during a chemical reaction.'
It means that the total mass of every element present in the products (RHS) of a chemical reaction has to be equal to their total mass present in the reactants (LHS). In other words, the number of atoms of each element remains the same, before and after a chemical reaction. In order to satisfy the law of conservation of mass, a chemical equation should be balanced.
Sodium + Water → Sodium hydroxide + Hydrogen (word equation)
Types of Chemical Reactions
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Types of Chemical Reactions
The chemical reactions are classified into different classes depending upon the types of chemical changes taking place. These reactions are as follows:-
- Combination Reaction
- Decombination Reaction
- Displacement Reaction
- Neutralisation Reaction
- Oxidation and Reduction Reactions
- Exothermic and Endothermic Reactions
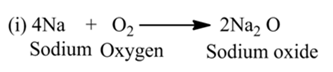
Combination Reaction
A reaction in which two or more reactants react together to form a single product is called a combination reaction.
For example,
(i):- When calcium oxide (quick lime) is dissolved in water, it forms calcium hydroxide (slaked lime).
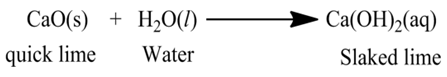
(iii):- Reaction between hydrogen gas and oxygen gas to form water.
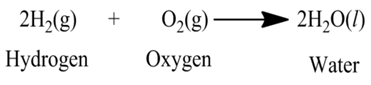
(iv):- Sulphur burns in air to form sulphur dioxide gas.
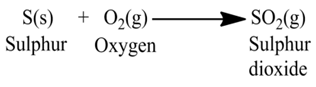
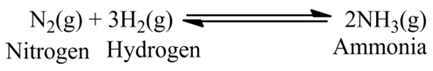
(v):- Nitrogen gas reacts with hydrogen gas to form ammonia gas.
Exothermic and Endothermic Reactions
(a) Exothermic Reactions:-
The reactions which are accompanied by the evolution of heat, are called exothermic reactions or the reactions in which heat is released alongwith the formation of products are called exothermic chemical reactions.
For example,
![]()
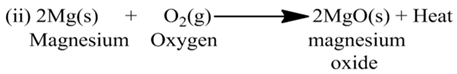
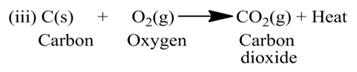
![]()
(b) Endothermic Reactions:-
The reaction, which occur by absorption of heat/energy are called endothermic reactions. It is also called hypothermic reaction. In other words, the energy needed for reaction to occur is less than the total energy released. As a result of this, the extra energy is absorbed in the form of heat.
For example,
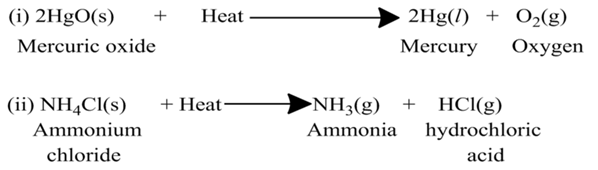

Decomposition Reaction
A reaction, in which a single reactant breaks down to form two or more products, is known as decomposition reaction. On the basis of the form of energy required for the reaction, these reactions are of three types:-
- Thermal Decomposition
- Electroylysis
- Photolysis or Photochemical Decomposition
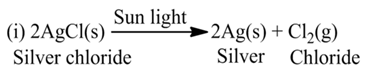
(a)Thermal Decomposition
These reactions use the energy in the form of heat for decomposition of the Reactant. For example,
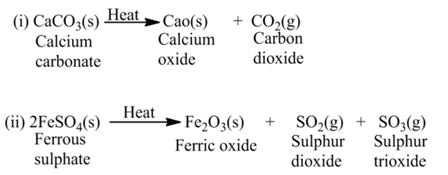
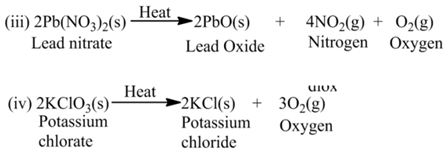
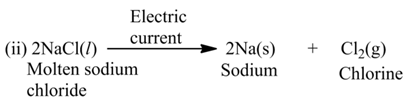
(b) Electrolysis
These reactions involve the use of electrical energy required for the decomposition of the reactant molecules. For example,
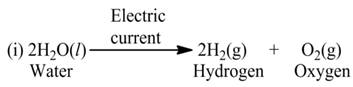
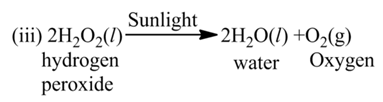
(c) Photolysis or Photochemical Decomposition
These reactions involve the use of light energy for the purpose of decomposition. For example,

Displacement Reaction

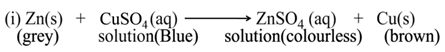
(a) Single Displacement Reaction
A reaction in which a more reactive element displaces a less reactive element from the solution of its compound, is called single displacement or displacement reaction.
For Example:-
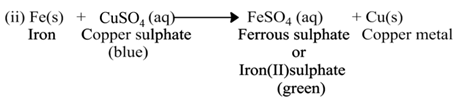
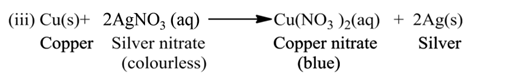
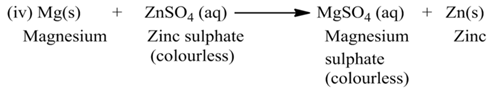
(b) Double Displacement Reaction
The reaction, in which two different ions in the reactant molecules are displaced by each other, is called double displacement reaction. For Example
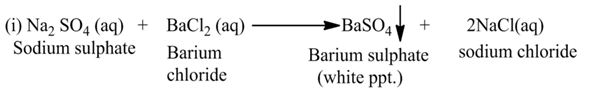

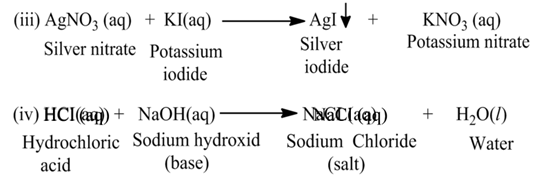
Neutralization Reaction
The reaction, in which an acid reacts with a base, is called neutralization reaction.
For Example:-
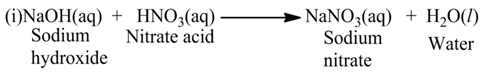
Oxidation and Reduction Reaction
OxidationThe process in which oxygen is added to a substances. OR The process in which hydrogen is removed from a substance.
For Example:-
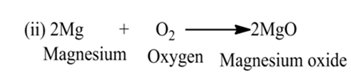
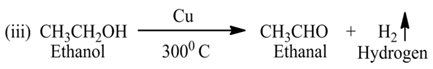
Here, ethanol is oxidized due to the removal of hydrogen.
Reduction
The process in which oxygen is removed from a substance. OR The process in which hydrogen is added to a substance.
For Example:-
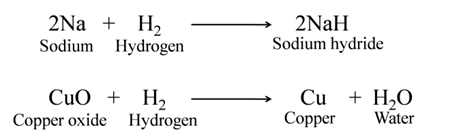
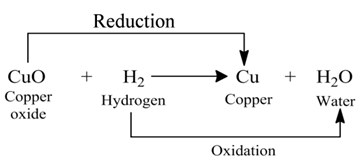
Redox Reactions
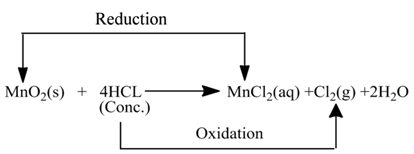
Those reactions in which oxidation and reduction take place simultaneously are called redox reactions.
(i):- In this reaction, the copper (II) oxide is losing oxygen and is being reduced. The hydrogen is gaining oxygen and is being oxidized.
(ii):- In this reaction HCl is oxidised toCl2, whereas MnO2 is reduced to MnCl2.
Oxidising Agent
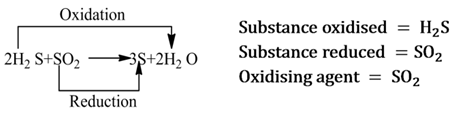
A substance which helps in the oxidation of another substance, is called oxidising agent. It either gives oxygen, removes hydrogen or accepts electrons from the substance to be oxidized.
Note:- It is always to be remembered that oxidising agent itself gets reduced.
Reducing Agent
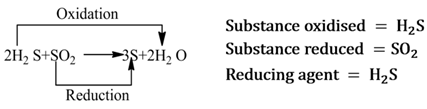
A substance that helps in the reduction of another substance, is called reducing agent. It either removes oxygen, gives hydrogen or donates electrons to the substance, that is reduced.
Note:- It is always to be remembered that reducing agent itself gets oxidised.
Effects of Oxidation Reaction in Everyday Life
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Effects of Oxidation Reaction in Everyday Life
Corrosion
The phenomenon due to which metals are slowly eaten away by the reaction of air, water and chemicals present in atmosphere, is called corrosion. Some examples of corrosion are:-
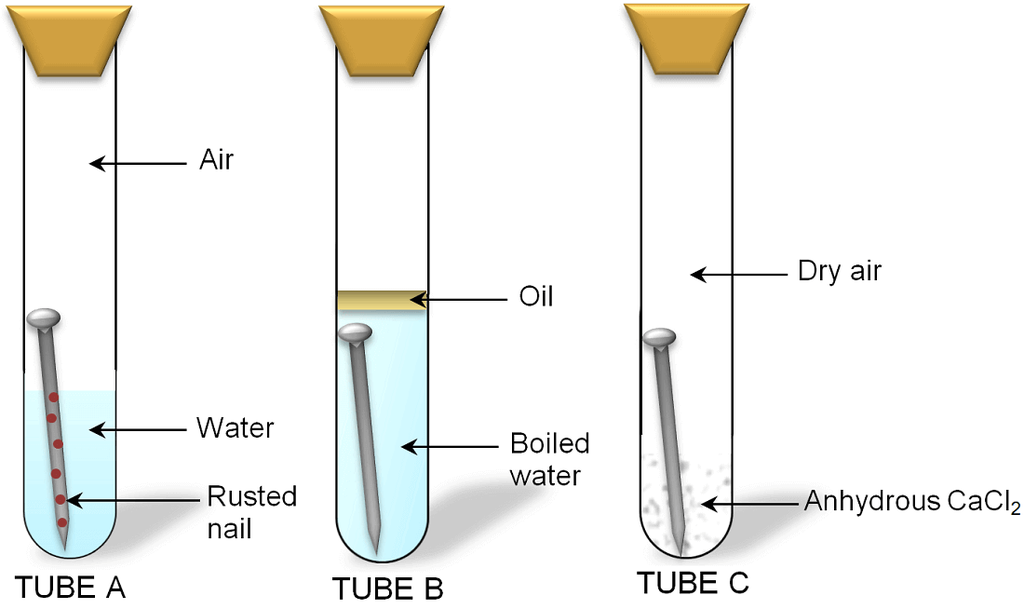
(i) Rusting of Iron Corrosion of iron is called rusting. Iron forms hydrated iron oxide (rust) when kept open in moist air.

(ii) Formation of a Green Layer Over Copper New copper (brown) vessel forms a green layer of basic copper carbonate after some time due to the reaction of air and moisture.
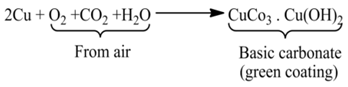
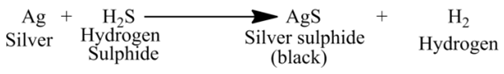
(iii) Tarnishing of Silver although silver is considered a noble metal, yet it reacts with some sulphur compounds as hydrogen sulphide to form black layer of silver sulphide.
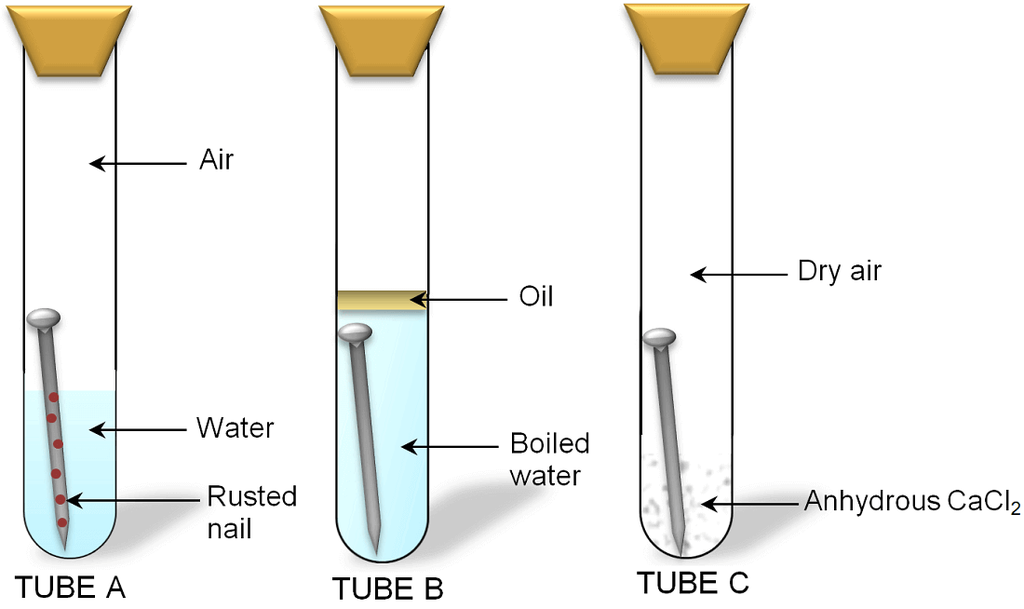
Conditions Necessary For Corrosion
The following two conditions are necessary for corrosion
- Presence of Air
- Presence of Water
Effects of Corrosion
Corrosion causes to damage to car bodies, bridges, iron railing, ships and all objects made of metals, specially those which are made of iron. Corrosion is a wasteful process in most of cases. Every year, a of tones of various metals especially iron get wasted in the country. Hence, it is quite necessary to prevent corrosion.
Exception In case of aluminium, corrosion is not proved to be wasteful. As aluminium is quite reactive metal, the top layer of it forms a thin layer of aluminium oxide which further prevents the corrosion of the metal. It is known as auto-protection mechanism.
Methods to Prevent Corrosion Iron
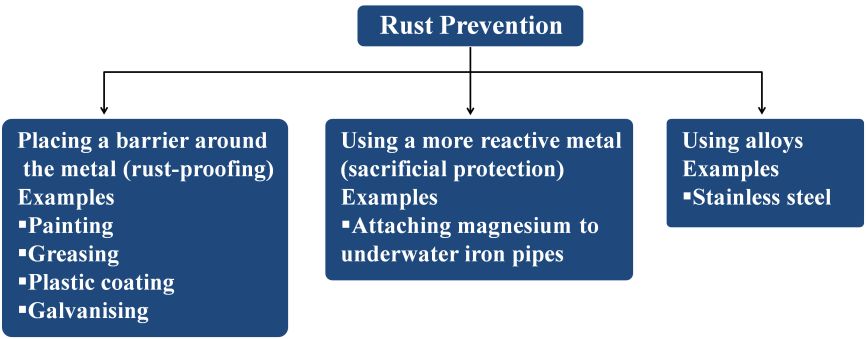
There are three genral methods to prevent corrosion of iron as shown below
Rancitidy
It is the process of slow oxidation of oil and fat present in the food materials resulting in the production of foul odour and taste in them. Conditions under which rancidity take places is when cooked food items are placed for a long time, they become rancid and unsuitable for consumption.
Methods to Prevent Rancidity
The methods to prevent rancidity are:-
- Packing of food materials in air tight containers.
- Refrigeration of cooked food at low temperatures.
- Packing of food items like potato wafers, etc in packets containing nitrogen gas instead of air.
- Avoiding the food materials and cooked food keeping in direct sunlight.
- By adding antioxidants. e.g., BHA (Butylated Hydroxy Anisole) and BHT (Butylated Hydroxy Toluene).
Introduction
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Chapter:- 2
Acids, Bases and Salts
Introduction
All the compounds on the basis of their chemical properties can be classified as acids, bases and salts. They have certain definite properties which distinguish one compound class from the other. The sour taste of many fruits and vegetables, (e.g., lemon, orange, amla, etc) is due to various types of acids present in them. The digestive fluids of most humans and animals contain acids. The bitter taste of substance like bitter gourd, cucumber extract, etc is due to bases present in them.
Acids
The word acid comes from the Latin word acidus acere which mean sour. Acids are those chemical substances which have a sour taste and turn blue litmus solution red. Some common fruits such as raw mango, lemon, orange, tamarind, etc are sour in taste.
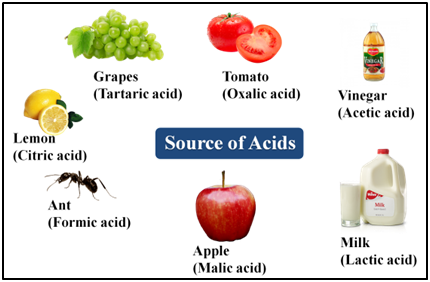
Classification of Acids
- On the Basis of Source
- On the Basis of Ionisaion
- On the Basis of the Amount/Concentration of Acid present in Aqueous Solution
On the Basis of Source
(i):- Inorganic Acids or Mineral Acids These are generally prepared from the minerals present in the earth's crust.
e.g., Hydrochloric acid (HCI), sulphuric acid (H2SO4), nitric acid (HNO3), carbonic acid (H2CO3), sulphurous acid (H2SO3), phosphoric acid (H3PO4), etc.
(ii):- Organic Acids or Edible Acids These are produced by plants or animals.
e.g., acetic acid (Source–vinegar), citric acid (Source–lemon, orange), maleic acid (Source–apple), lactic acid (Source–curd), tartaric acid (Source–tamarind), oxalic acid (Source–tomato), methanoic acid (Source–ant sting, nettle sting), etc.
On the Basis of Ionisation
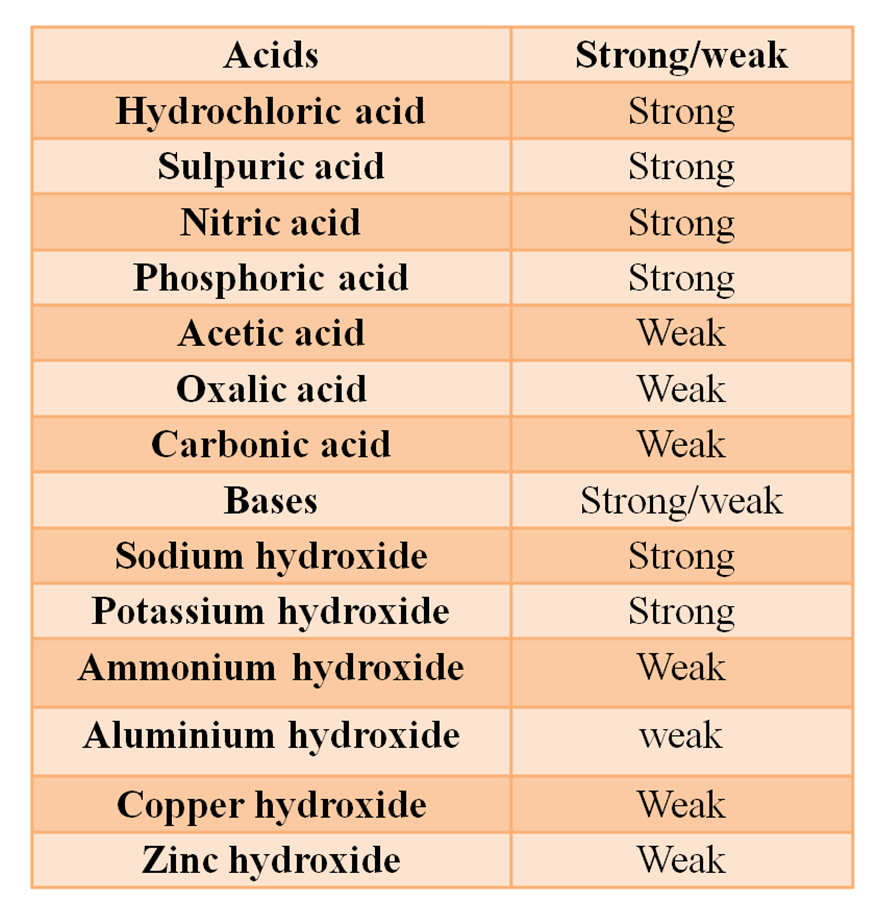
(i):- Strong Acid The acids which ionize almost completely are called strong acids. e.g., mineral acids (except H2CO3).
(ii):- Weak Acid The acids which ionize only partially or to a lesser extent are called weak acids. e.g., organic acids.
On the Basis of the Amount/Concentration of Acid present in Aqueous Solution
(i):- Dilute Acid If in an aqueous solution. Concentration of acid is low, it is called dilute acid.
(ii):- Concentrated Acid If concentration of acid is high, it is called concentrated.
Bases
Substance that furnish hydroxide ions (OH–) in aqueous solution are called bases. Bases are bitter in taste and soapy to touch and turn red litmus solution blue. Their examples are caustic soda or sodium hydroxide NaOH, calcium hydroxide Ca(OH)2, caustic potash or potassium hydroxide KOH, milk of magnesia or magnesium hydroxide Mg(OH)2, etc.
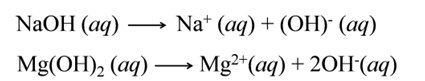
Types of Bases
Bases are of two types:-
(i):- Strong Bases The substances/bases which ionise completely to furnish OH- ions are called strong bases. e.g.,
![]()
(ii):- Weak Bases The bases which ionise only partially are called weak bases. e.g.,
![]()
Alkali
Water soluble base is called alkali. eg., NaOH, KOH, etc. These are usually in laboratory as aqueous solution. These are soapy to touch, bitter and corrosive. Never taste them as they may cause harm. All alkalies are base, but all base, but all base are not alklies.
Strong/Weak Acids and Bases are Tabulated Below
Chemical Properties of Acids
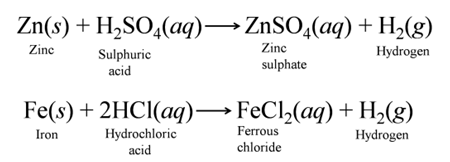
(a) Reaction with Metals Dilute acids (e.g., HCl and H2SO4 not HNO3) react with certain active metals like zinc (Zn), iron (Fe), etc to evolve H2, gas. For Example:-
Test for H2 Gas When a burning match stick is brought near the hydrogen gas, it burns with pop sound.
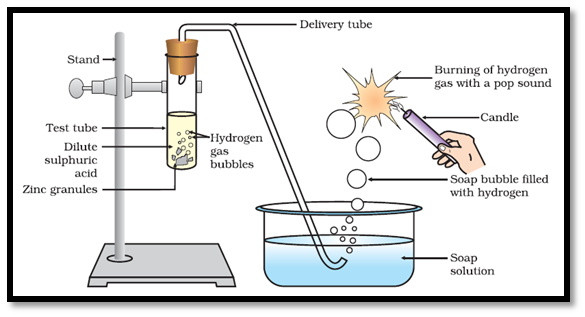
Metal Oxides
When metal reacts with O2 and H2O present in atmosphere it produces metal oxides. Metal oxides are base in nature.
(b) Reaction with Metal Oxides Acids react with certain metal oxides (basic oxides) to form salt and water. For Example:-
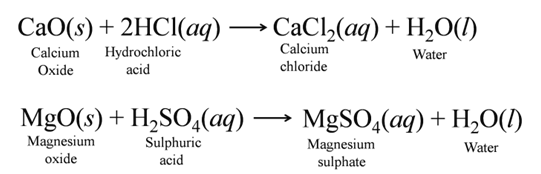
(c) Reaction with Metal Carbonate and Hydrogen Carbonate Acids react with metal carbonates and hydrogen carbonates to produce carbon dioxide gas. 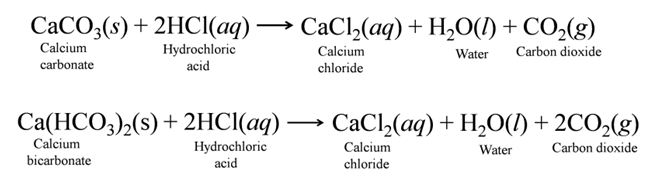
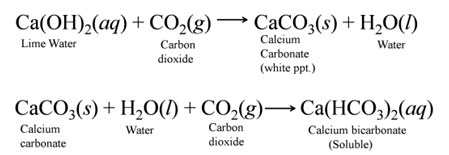
Test for , Gas When gas is passed through lime water, it turns milky due to the formation of white ppt. of CaCO3. But if CO2 is passed in excess, milkiness disappears due to the formation of CaCH(O3)2 which is soluble in water. For Example:-
Chemical Properties of Bases
(a) Reaction with Metals Strong bases react with active metals to produce hydrogen gas.

(b) Reaction with Non-metallic Oxide Bases react with non-metallic oxides (acidic oxides) to produce salt and water. This reaction proves that non-metallic oxides are acidic in nature.
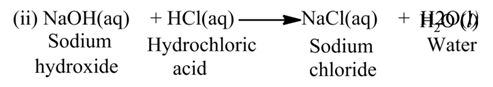
Reaction Between Acids and Bases
Acids react with bases to produce salt and water. In this reaction, acid neutralize a base and the reaction is called neutralization reaction. i.e., reduce its effect or vice-versa, thus the reaction is known as neutralisation reaction.

Common Characteristics of Acids and Bases
Both acids and bases conduct free electric current in their aqueous solutions due to the presence of free ions.
Effect of Dilution on an Acid or Base
Mixing of an acid or base with water is called dilution. It results in decrease in the concentration of ions (H3O+/OH–) per unit volume and the acid or base is said to be diluted.
When water is added to an acid or base, their molecules dissociate to form ions. The H+ ions
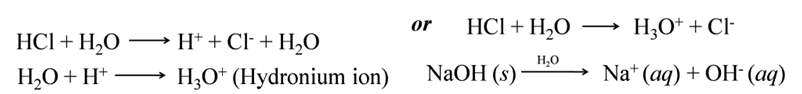
Note:- The process of dissolving an acid or base in water is a highly exothermic reaction. If water is added to a concentrated acid, the heat generated may cause the mixture to splash out and cause burns. To avoid an accident, acid (or base) should be slowly added to the container containing water along with its sides with constant stirring.
Uses of Acids
- Vinegar (acetic acid) is used to preserve pickles and Chinese food.
- In stomach, hydrochloric acid is released which makes the medium acidic and leads to coagulation of proteins. It also helps in digestion and kill the bacteria coming to the stomach along with the food.
- Cold drinks contain carbonic acid.
- Oxalic acid is used to remove ink or rust stains.
- Boric acid is used as an antiseptic and for eye wash.
- Sodium benzoate is used as a preservative in cold drinks.
- Oranges and amla contain ascorbic acid (vitamin C) which prevents scurvy.
Indicators
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Indicators
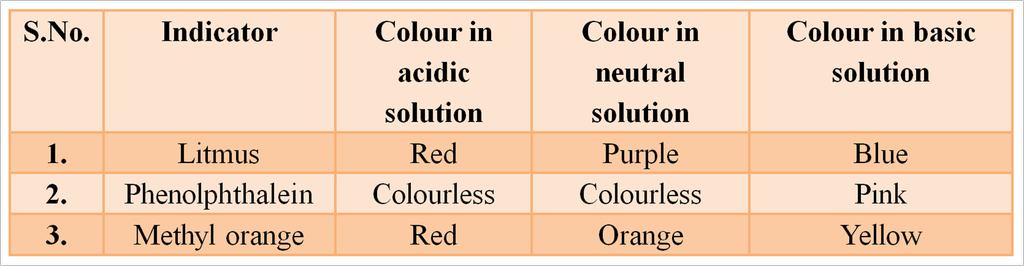
Indicators
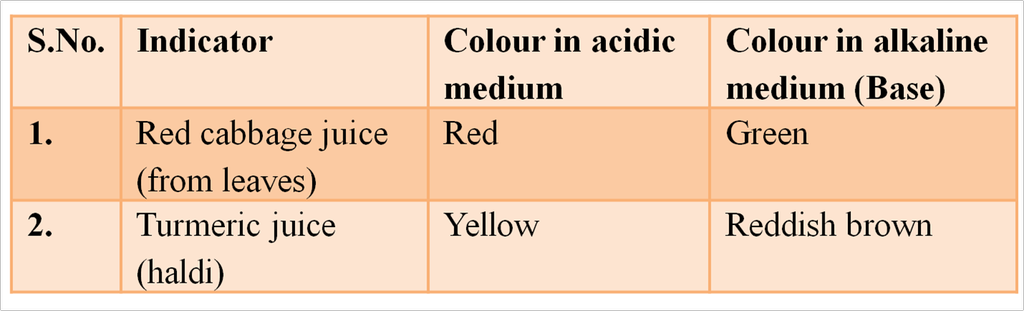
The easiest way to detect whether a solution is acidic, alkaline or neutral is to use an indicator. Indicators are substances that change their colour or odour if they are put into an acid or alkaline solution. Litmus, turmeric and China rose extract, etc are natural indicators.
Some Natural Indicators with Characteristic Colour
Synthetic Indicators
Those chemical substances which change their colour in acids and bases are called synthetic indicators.
They are also called synthetic acid base indicators. e.g., methyl orange, phenolphthalein methylene blue, and methyl red are synthetic indicator.
Some Synthetic Indicators with Characteristic Colour
Olfactory Indicators
Those substances whose odour changes in acidic or basic media are called olfactory indicators.
Vanilla, onion and clove can be used as olfactory indicator.
Litmus Indicators
It is purple dye, which is extracted from lichen, a plant from the division Thallophyta. It is commonly used as acid-base indicator. When the litmus solution is neither acidic nor basic, its colour is purple. When purple solution is acidified, it changes to blue colour whereas it changes to red colour when a small amount of base is added to blue litmus solution.
Strength of an Acid or Base
Strength of an acid or base depends on the number of H+ ions or OH- ions produced by them respectively.
Larger number the of H+ ions produced by an acid, stronger is the acid. Similarly, larger the number the OH- ions produced by a base, stronger is the base.
To judge how strong a given acid or base is, a universal indicator is used which is mixture of several indicators. It shows different colours at different concentrations of hydrogen ion in a solution.
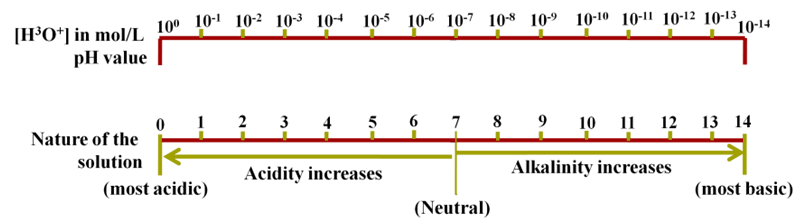
The pH Scale
It is a scale used for measuring hydrogen ion concentration. The p in the pH stands for potenz which means power in German. It has values zero (very acidic) to fourteen (very alkaline).
pH is number which indicates the acidic or basic nature of a solution. Higher the hydronium ion concentration present in the solution lower is their pH value.
{pH means power of hydrogen ions}
- If pH > 7, solution is basic
- If pH < 7, solution is acidic
- If pH = 7, solution is neutral
Importance of pH in Everyday Life
Following are the examples showing importance of pH in everyday life
(i):- pH of Soil Every type of plant requires a specific pH range for healthy growth. Therefore, the nature of soil is known first by testing its pH and then a particular crop is grown in it. It is also suitable for selecting the fertiliser for a particular crop by knowing the pH of he soil.
(ii):- Effect pH on our Digestion HCl present in the stomach helps in the digestion of food. When acidity increases in the stomach, pain, irritation and indigestion is caused. To correct the disturbed pH range, milk of magnesia is used as a medicine, which is also called antacid as it reduces the
effect of acid (or acidity).
(iii):- Effect of pH in Tooth Decay Tooth enamel is made up of calcium phosphate and is the hardest substance in the body. If the pH inside the mouth decreases than 5.5, the decay of tooth enamel begins. This happens when the bacteria present in the mouth work on the left over food particles and produce acid. To prevent tooth decay toothpastes (basic) are used which neutralise the excess acid.
(iv):- When insects like honeybee, ant, etc bite; they leave an acid in the skin, that causes pain and irritation. If a mild base like baking soda is applied on the affected area, it gives relief.
(v):- Stinging Hair of Neetle leaves injects methanoic acid in the skin which causes pain. It is cured by rubbing the affected area with the leaves of dock plant, found in the same locality where the neetle plant is found.
Salts
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Salts
Salts are produced by the neutralisation reaction between acid and base.
![]()

Here X is non – metal and M is metal i.e., we can say when metal ion takes place of H - ions, then salt is formed.
Family of Salts
Salts having the same positive or negative radicals are said to belong to a family. For Example:-
NaCl and Na2SO4 belong to the family of sodium salts. Similarly, NaCl and KCl belong to the family of chloride salts.
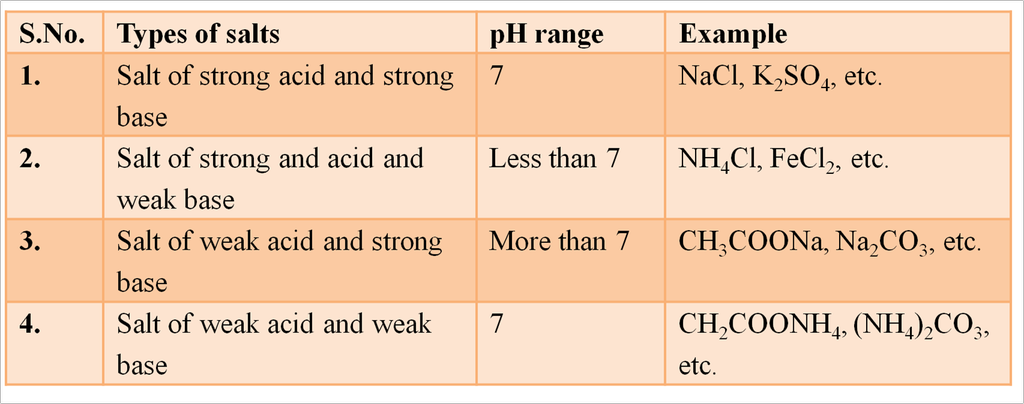
pH of Salts
- Salts of strong acid and a strong base are neutral with pH value of 7.
- Salts of a strong acid and weak base are acidic with pH value less than 7.
- Salts of strong base and weak acid are basic in nature with pH value more than7.
Common Salt: Sodium Chloride
Common salt is formed by the combination of hydrochloric acid and sodium hydroxide solution. It is the salt that we use in food.
It is obtained on large scale from sea water by separating other salts from it. It may also be obtained from rock salt.
Uses of Sodium Chloride
- Common salt, apart from cooking food, is used as a preservative.
- It is also used for manufacture of Na metal and Cl2 (g) by electrolysis in molten state.
- The common salt is an important raw material for various materials of daily use, such as sodium hydroxide, baking soda, washing soda, bleaching power and many more.
Caustic Soda (Sodium Hydroxide [NaOH])
When electricity is passed through an aqueous solution of sodium chloride (called brine), it decomposes to form sodium hydroxide. The process is called the chlor-alkali process because of the products formed chlor for chlorine and alkali for sodium hydroxide.
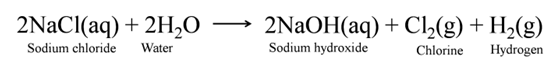
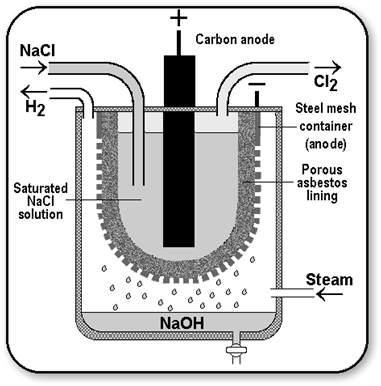
Uses of Hydrogen
- Hydrogen is used in the hydrogen of oils to obtain solid fats. (Vegetable ghee).
- It is used to make ammonia for fertilizers, methanol and production of hydrochloric acid. Hydrochloric acid is used in the preparation of ammonium chlorides, in medicines and cosmetics.
- Liquid hydrogen is used as a fuel for rockets.
Uses of Chlorine
- Chlorine is used to sterlise drinking water supply and the water in swimming pools.
- It is used in the production of bleaching powder, Poly Vinyl Chloride (PVC) pesticides, chloroform, paints and dyestuff.
Uses of Caustic Soda
- It is used for making soaps and detergents.
- It is used in the production of bleaching powder, Poly Vinyl Chloride (PVC) pesticides, chloroform, paints and dyestuff.
Bleaching Powder
(Calcium Oxychloride [CaOCl2])
It is produced by the action of chlorine on dry slaked lime.
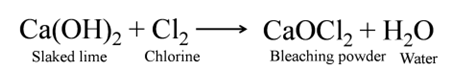
On standing for a longer time, it undergoes auto-oxidation due to which bleaching action decreases. Hypo or sodium bisulphite is used as antichlor during bleaching action.
Uses of Bleaching Powder
- It is used for bleaching purposes in textile and paper industry and in laundry.
- It is also used as a disinfectant for water.
- It is used as an oxidizing agent too.
- It is used in the manufacture of chloroform.
Baking Soda
(Sodium Hydrogen Carbonate or Sodium Bicarbonate [NaHCO3])
The soda commonly used in the kitchen for making tasty crispy pakoras is baking soda. It is the major constituent of baking powder. Sometimes it is added for faster cooking. Chemically it is sodium hydrogen carbonate. It is produced using sodium chloride as one of the raw materials.
Manufacture of baking soda is shown in reaction below
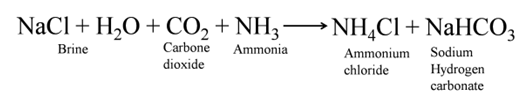
Uses of Baking Soda
For making baking powder, which is a mixture of baking soda (sodium hydrogen carbonate) and a mild edible acid such as tartaric acid. When baking powder is heated or mixed in water, the following reaction takes place
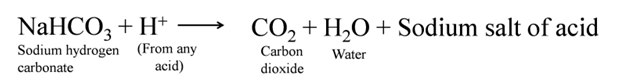
Carbon dioxide produced during the reaction causes bread or cake to rise making them soft and spongy.
Sodium hydrogen carbonate is also an ingredient in antacids. Being alkaline, it neutralises excess acid in the stomach and provides relief.

It is also used in soda-acid fire extinguishers
Washing Soda
(Sodium Carbonate [Na2CO3.10H2O])
Sodium Carbonate can be obtained by heating baking soda; recrystallisation of sodium carbonate gives washing soda. It is also a basic salt.
![]()
Uses of Washing Soda
- It is used in glass, soap and paper industry.
- It is used for the manufacture of sodium compounds like borax.
- It also removes permanent hardness of water.
- It is used as cleansing agent (detergent) in houses and laundries.
Plaster of Paris
(Calcium Sulphate Hemihydrate [CaSO4.1/2H2O])
It is obtained by heating gypsum (CaSO4.2H2O) at 373 K. At this temperature, gypsum loses water molecules and forms plaster of paris.
![]()
When gypsum is heated above 400K, dead burnt plaster (anhydrous CaSO4) is obtained which does not have the property of hardening.
Uses of Plaster Paris
- It is used by doctors for joining the fractured bones in right position i.e., for making plaster to support fractured bones.
- POP made by it is also used for making decorative pieces and for making designs on ceilings.
Water of Crystallization
Crystals of some compounds seem to be dry (or anhydrous), but actually contain some water molecules attached to them. This water is called water of crystallization and such salts are called hydrated salts. Water of crystallization is the fixed number of water molecules present in one formula unit of a salt. For Example:-
- Hydrated copper sulphate (blue vitriol) CaSO4.5H2O
- Hydrated ferrous sulphate (green vitriol) FeSO4.7H2O
- Washing soda crystals Na2CO3.10H2O
This water is removed by heating the crystal of the hydrated salt.
Metals
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Chapter:- 3
Metals and Non-metals
Introduction
About 118 chemical elements are known at present. On the basis of their properties, all the elements can be divided into two main groups i.e., metals and non-metals. Apart from metals and non-metals, some elements show properties of both metals and non-metals. These are called metalloids.
Both metals and non-metals are very important in our everyday life. We also use a large number of compounds of metals and non-metals.
Metals
Elements that are electropositive in nature are called metals. They have a tendency to lose electrons and form positive ions. For example, copper, iron, aluminium, sodium, etc.

Physical Properties of Metals
- Malleability It is the property of metal due to which it can be beaten into thin sheets.
- Ductility It is the property due to which a metal can be drawn into wires.
- Hardness Most of the metals are hard. But some alkali metals like sodium and potassium are so soft that they can be cut easily with knife.
- Metallic Lustre Metals in their pure state have bright shining surfaces.
- Electrical Conductivity Most of the metals are good conductors of electricity.
- Thermal Conductivity Generally metals are good conductors of heat, except lead and mercury.
- Melting and Boiling Points Metals generally have high melting and boiling point, except mercury, gallium and alkali metals.
- Sonority When metals are struck with a hard substance, they produce sound.
Chemical Properties of Metals
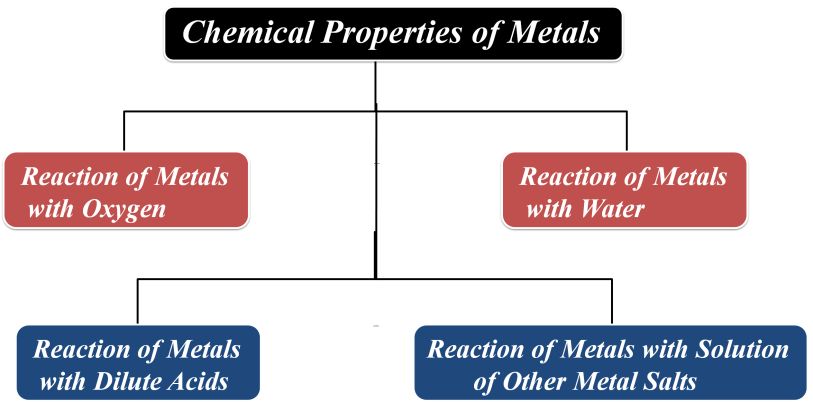
Most of the chemical properties of metals are due to their electropositive nature. It means that a metal atom lose electrons to form cations.
Reaction of Metals with Oxygen
Burning in Air (Formation of oxides)
Almost all the metals react with oxygen (or air) to form metal oxides.
![]()
For example, when copper is heated in air, it combines with oxygen to form copper (II) oxide, a black oxide.
![]()
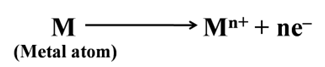
Similarly, sodium forms sodium oxide
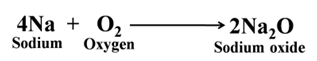
Magnesium forms magnesium oxide
Aluminium forms aluminium oxide
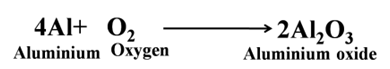
Nature of Metallic Oxides
(Formation of hydroxides)
Most of the metallic oxides are basic in nature. When dissolved in water, they produce alkali. For Example:-
Similarly, sodium oxide forms sodium hydroxide
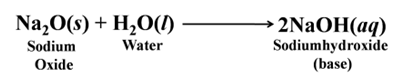
Note:- Alkalis are the bases that dissolve in water.
Exception
Some metal oxides show both types of behavior, i.e., acidic and basic. These oxides are called amphoteric oxides. e.g., zinc oxide, aluminium oxide, etc. For Example:-
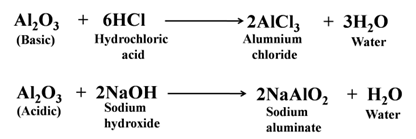
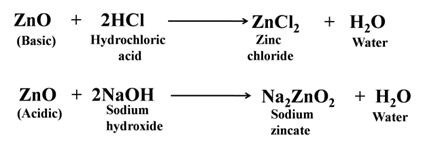
Order of Reactivity with Oxygen
Different metals react with oxygen at different rates. For example, sodium (Na) and potassium (K) catch fire, when placed in the open. Hence, these are most reactive metals. To prevent accidental fires, these metals are kept immersed in kerosene oil. Magnesium (Mg) burns in air only by heating. Zinc (Zn) burns only on strong heating while iron does not burn in the form of rod or block but burns in the form of filings. Copper (Cu) does not burn on heating but blister copper burns.
Oxide silver does not react with oxygen. Hence, the order of reactivity of these metals with oxygen is Na > Mg > Zn > Fe > Cu > Ag.
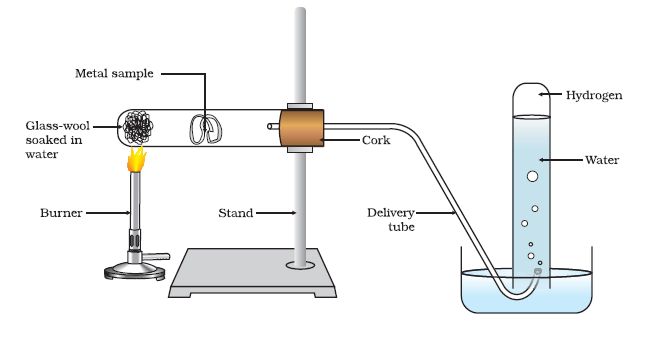
Reaction of Metals with Water
Metals react with water and produce a metal oxide and hydrogen gas. Metals oxides that are soluble in water dissolve into further to form metal hydroxides but all metals do not react with water.
Metals like potassium and sodium react violently with cold water. In case of Na and K, the reaction is very violent and exothermic.
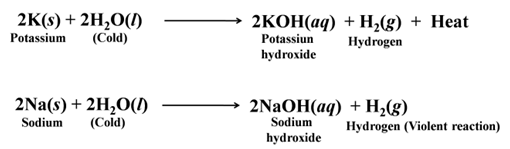
The, heat evolved is sufficient for hydrogen to catch fire. That’s why Na and K catch fire when kept in water.
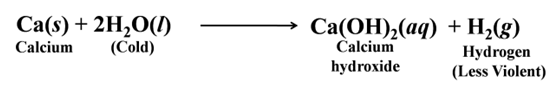
The reaction of calcium with water is less violent. The heat evolved is not sufficient for the hydrogen to catch fire.
Magnesium does not react with cold water. It reacts with hot water and forms magnesium hydroxide and hydrogen.
It also starts floating due to the bubbles of hydrogen gas sticking to its surface.
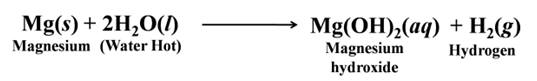
Metals like aluminium, iron and zinc do not react either with cold or hot water. They react with steam and form the metal oxide and hydrogen.
Lead, copper, silver and gold do not react with water at all. Thus, the reactivity order of metals towards water is
K > Na > Ca > Mg > Al > Fe > Pb > Cu > Ag > Au.
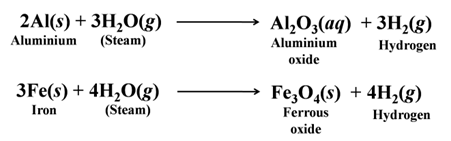
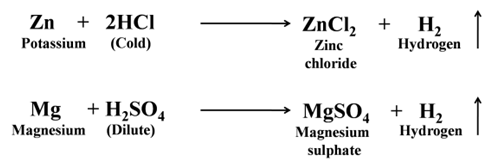
Reaction of Metals with Dilute Acids
- Reaction of Metals with Dilute HCl and Dilute H2SO4.
Except a few less reactive metals (Cu, Hg, Ag, Au, Pt, etc) all metals react with dilute sulphuric acid and hydrochloric acid to produce salt and hydrogen gas. For Example:-
Reaction of Metals with Dilute HNO3 Except magnesium and manganese, metals do not react with dilute nitric acid. This is due to the oxidising nature of nitric acid.
Normal Case:-
![]()
![]()
Exceptional Case (for Mn and Mg only)
Aqua Regia
It is a freshly prepared mixture of concentrated hydrochloric acid and concentrated nitric acid in the ratio of 3 : 1. It can dissolve gold, even though neither of these acids can do so alone.
Aqua regia is a highly corrosive, fuming liquid; It is one of the few reagents that is able to dissolve gold and platinum.
Reaction of Metals with Solution of Other Metal Salts
A reactive metal can displace a comparatively less reactive metal from their compounds in aqueous solution or in molten state.
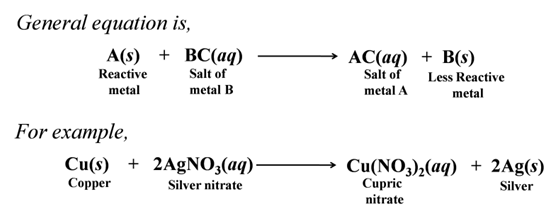
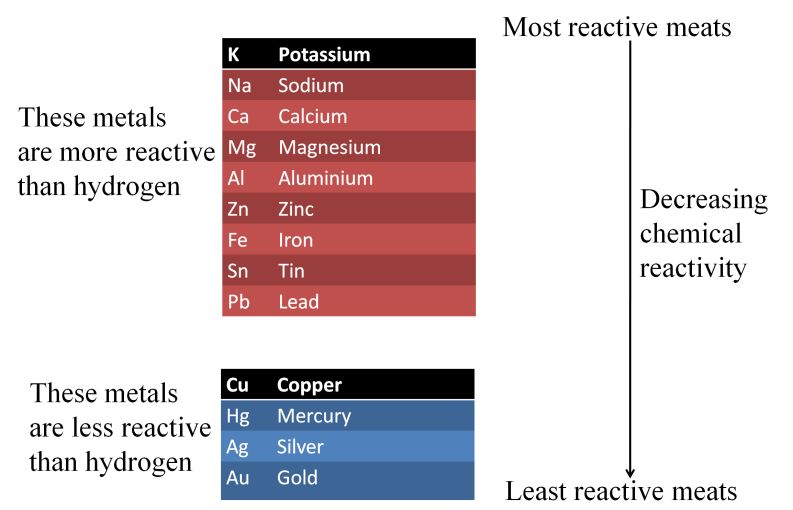
Reactivity Series or Activity Series of Metals
The arrangement of metals in the order of their decreasing reactivities is called reactivity series of metals.
Importance of Reactivity Series
The following two points state importance of reactivity series
- In reactivity series, metals that are placed above hydrogen, can displace hydrogen from dilute acids (HCI and H2SO4).
- Metal placed above another metal in this series can displace it from the solutions of its salt, as the metal towards top is more reactive than a metal placed below it.
Non-Metals
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Non-Metals
Elements that are electronegative in nature are called non-metals. It means non-metals gain electrons to form negative ions.
For Example, iodine, sulphur, hydrogen, etc.

Physical Properties of Non-Metals
- Brittleness Non-metals are neither malleable nor ductile but they are brittle in nature.
- Physical State Most of the non-metals are soft (if solid). Only diamond, a form of carbon is the hardest known substance. Other non-metals are gases except bromine which is a liquid.
- Metallic Lustre the non-metals do not have lustre. However, diamond, iodine and graphite, have lustre.
- Electrical and Thermal Conductivity Non-metals are generally poor conductors of heat and electricity except graphite.
- Melting and Boiling Points Generally, non-metals have low melting and boiling points. But non-metals that are solids have comparatively higher boiling points. (e.g., B. Si, C, etc.)
Chemical Properties of Non-Metals
- Reaction with Oxygen Non-metals react with oxygen to form oxides. These oxides are generally acidic. Only some of the non-metallic oxides are neutral.
Acidic oxides are CO2, SO2, P2O5, etc. For Example:-
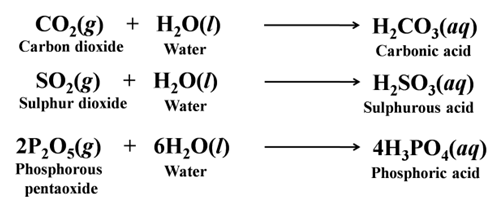
Neutral oxides are CO, H2O, N2O, etc.
Reaction with Water Non-metals do not react with water or steam to evolve hydrogen gas. This is because non-metals cannot give electrons to hydrogen in water therefore, hydrogen gas cannot be released.
Reaction with Acids Non-metals do not react with acids to release hydrogen gas. Reason is that non-metals cannot loose electrons and give it to hydrogen ions of acids so, that the gas is released.
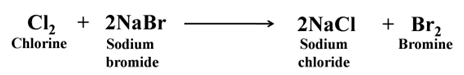
Displacement Reaction Non-metals also show displacement reaction like metals. For Example:-
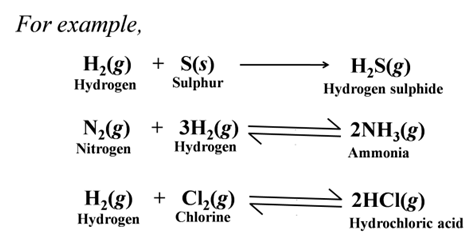
Formation of Covalent Compounds Non-metals form covalent compounds with other non-metals like hydrides, chlorides, etc.
Exceptions Among Metals and Non-metals
- All metals (except mercury, a liquid metal) exist in solid state at normal room temperature.
- Gallium and cesium, (metals) have very low melting point. When kept on the palm, they start melting.
- Iodine, diamond and graphite are lustrous although they are non-metals.
- Diamond has a very high melting point and is very hard although it is a non-metal.
- Alkali metals have Low densities and low melting points. They are too soft to be cut with a knife.
- Graphite is a good conductor of electricity although it is non-metal.
Reaction of Metals and Non-Metals
Formation of Ionic Compounds
We know that noble gases are unreactive and the reason for their unreactive nature is the presence of 8 electrons in their outermost shell (octet) with an exception of helium (which has duplet i.e., two electrons in the outermost or the only shell).
The elements try to get 8 electrons in their outermost shell as it is the state of maximum stability or
minimum energy (by gaining or losing electrons).
To recall them, see the following table:-

Formation of Sodium Chloride
The two oppositely charged ions (sodium and chloride ions in the below case) bind together with the help of strong electrostatic forces of attraction which is termed as ionic bond or electrovalent bond and the compounds having it are called ionic or electrovalent compounds.
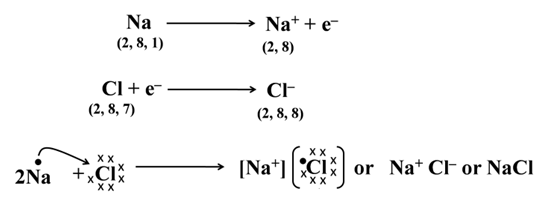
Thus, it is also clear that ionic compounds (like sodium chloride) do not exist as discrete molecules but indeed they are the aggregates of oppositely charged particles.
Formation of Calcium Chloride
Formation of ionic bond, can further be understood by taking an example of calcium chloride (where calcium is a metal and chlorine is a non-metal), in which calcium (Ca) with configuration K, L, M, N have a tendency to lose teo electrons and chlorine with configuration K, L, M have a tendency to gain one electron.
Thus, the two electrons lost by Ca are achieved by two Cl atoms. The Ca atom acquires two positive charges (Ca2+) and each Cl atom acquires a negative charge (Cl–). These oppositely charged ions are held together by electrostatic forces i.e., by ionic bond.
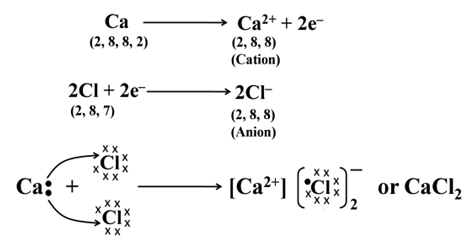
Properties of Ionic Compounds
- Physical Nature Ionic compounds are crystalline solids.
- Melting and Boiling Points These compounds have high melting and boiling points as large amount of energy is required to break strong forces of attraction.
- Solubility These compounds are soluble in water and insoluble in organic solvents like kerosene, benzene, ether, petrol, etc.
- Conduction of Electricity Ionic compounds are good conductors of electricity, but they conduct electricity either in molten state or in their aqueous solution.
Occurrence and Extraction of Metals
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Occurrence and Extraction of Metals
The earth's crust is the major source of metals. Sea water is also a source for some metals. It contains soluble salts like sodium chloride, magnesium chloride, etc.
Minerals The naturally occurring elements or compounds of metal present in the earth’s crust are called minerals.
Ores Those minerals from which a particular metal be extracted profitably are called ores. Each ore is a mineral but each mineral is not an ore.
Gangue the undesirable impurities like soil, sand, etc found in ore are called gangue.
Enrichment of Ores Removal of unwanted material (gangue)
Extraction of Metals
Attainment of pure metal from its compound is called extraction of metals. Some metals are found in earth’s crust in Free State while some are found in the form of their compounds. The metals present at the bottom of the reactivity series are least reactive so they are found in Free State. e.g., Gold, Silver, Platinum, Copper.
The metals at the top of the reactivity series are highly reactive so they are found as free elements. The metals in the middle of the reactivity series (zinc, iron, lead) are moderately reactive, found as oxides, sulphides or carbonates.Copper and silver are found in the combined state in their sulphide or oxide ores.
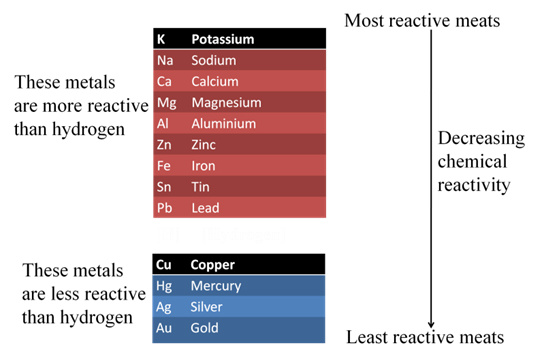
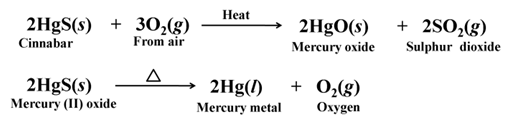
Extracting Metals Low in the Activity Series (Low in activity series)
Cinnabar (HgS) is an ore of mercury, when heated in air, it first changes into its oxide and then into mercury metal.
For example,
Copper glance (Cu2S) when heated in air, partially oxidised and the oxidised product reacts with the remaining copper glance to give copper metal.
For example,
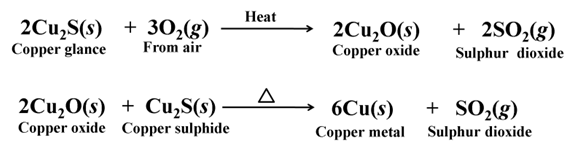
Extraction of Metals in the Middle of the Activity Series
The metals in the middle of the activity series are usually present as sulphides or carbonates in nature. Sulphides are converted into oxides by roasting and carbonates are converted into oxides by calcination
Roasting

Calcination

Reducation
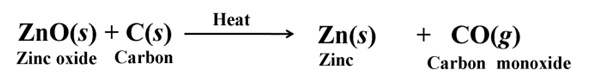
Thermit Reaction
The reaction of iron (III) oxide (Fe2O3) with aluminium is used to join railway tracks or cracked machine parts of heavy machines.
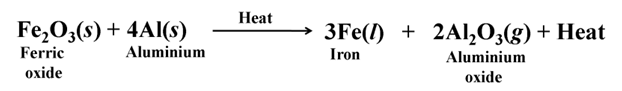
This reaction is known as thermit reaction its highly exothermic.
Extraction of Metals Towards the Top in Activity Series
These metallic compounds cannot be reduced by carbon or any other reducing agent due to their high affinity with oxygen. Therefore, electrolytic reduction is employed for these metals e.g., Na, Mg, Ca, etc.
Electrolytic reduction the salts of these metals as chlorides in molten form are electrolysed. Metal is deposited at the cathode and chlorine is liberated at the anode.
The reactions are (At cathode)
![]()
The reactions are (At anode)
![]()
Refining of Metals
It is the process of purification of the metal obtained after reduction. Various methods for refining are employed, but the most common one is the electrolytic refining.
Electrolytic Refining
Many metals like Cu, Sn, Ni, Ag, Au, etc are refined electrolytically.
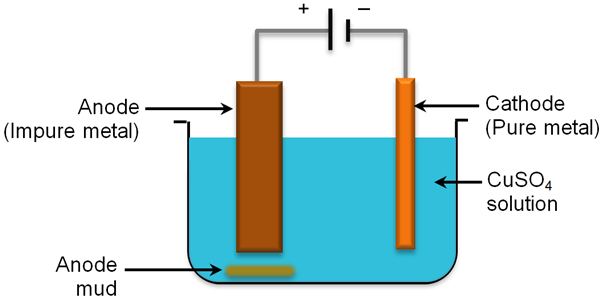
The reactions are (At cathode)

The reactions are (At anode)
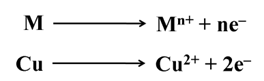
Corrosion
It is the process of slow eating away of metals by the reaction of atmospheric air and moisture.For example, rusting of iron, tarnishing of silver, formation of green coating over copper etc.

Prevention of Corrosion
Rusting of iron is prevented by painting, oiling, greasing, galvanising, chrome plating, anodising and by making alloys.
Galvanisation
The process of coating iron and steel objects with a thin layer of zinc is called galvanisation. It is done by dipping the object in molten zinc.

Anodising
It is the process of forming a thick oxide layer on the surface of metal. At ordinary temperature the surfaces of metals like zinc, aluminium, etc are covered with a thin layer of oxide. This oxide layer is protective and prevents the metal from further oxidation. This layer can be made more thick by anodising.
In this process. Clean metal is taken as anode and dilute H2SO4 as electrolyte. When electric current is passed. O2 gas is liberated which reacts with metal to form a layer of oxide.
Alloy
An alloy is a homogeneous mixture of two or more metals or a metal and a non-metal. It is prepared by mixing the metals in molten state and then cooling the mixture. The electrical conductivity and melting point of an alloy is less than that of pure metals.
For example,
- Amalgam If an alloy contains mercury as one of its components, it is called amalgam.
- Alloying of Gold Pure gold is very soft. It is called 24 carat gold. To increase the strength and hardness of gold and to make it suitable for making jewellery, alloy of gold is made with either silver or copper.
- Alloying of Iron Pure iron is very soft and stretches easily when hot. It is mixed with a small amount of carbon (about 0.05%) and it becomes strong.
Alloying
It is the method of improving the properties of a metal by mixing two or more metals

Purpose of Making Alloy
- Alloy do not corrode or corrode to very less extent.
- Alloy is much harder and stronger than the pure metal.
- Alloy has less conductance than pure metal.
- Some alloys have, low melting point and hence used for soldering of metals.
Alloys, their Composition and Uses
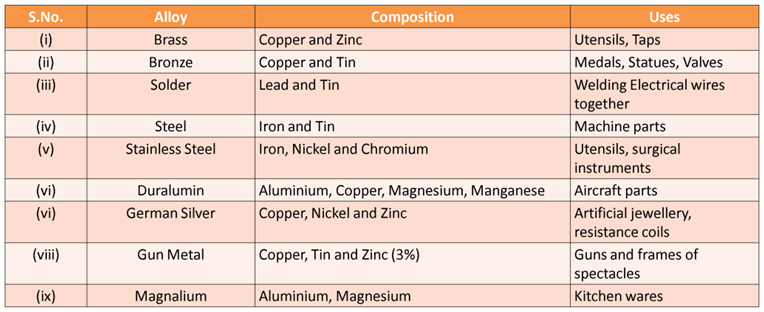
Constituents and Uses of Alloys
Brass (copper and zinc) it is used in making utensils and taps.
Bronze (copper and tin) it is used in making medals, statues, etc.
Solder (lead and tin) it is used to weld electrical wires.
Carbon and Its Compounds
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Chapter:- 4
Carbon and its Compounds
Carbon and Its Compounds
Carbon is considered as the third most important element, after oxygen and hydrogen, for the existence of life on the earth.
The Covalent Bond
![]()
Thus, there are 4 electrons in its outermost shell and its octet can be completed by the following two ways
- It could lose 4 electrons and form C4+ cation. But a massive amount of energy is required to remove 4 electrons leaving behind a carbon cation with 6 protons in its nucleus holding on just two electrons together.
- It could gain 4 electrons and can form C4- anion. But for a nucleus having 6 protons, it would be difficult to hold on 10 electrons, i.e. 4 extra electrons.
In order to overcome this problem, carbon shares its valence electrons with other atoms of itself or atoms of other elements.
- This type of bonding is called covalent bonding.
- The bonds which are formed by the sharing of an electron pair between two same or different atoms are known as covalent bonds.
- The number of electrons shared show the covalency of that atom.
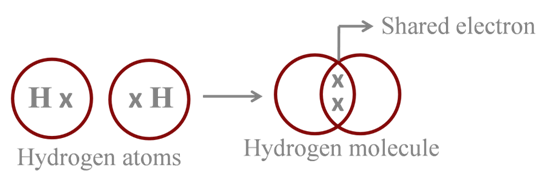
Formation of Hydrogen Molecule
Atomic number of H = 1
![]()
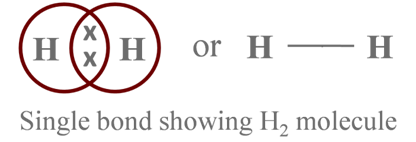
The shared pair of electrons constitutes a single bond between the two H-atoms
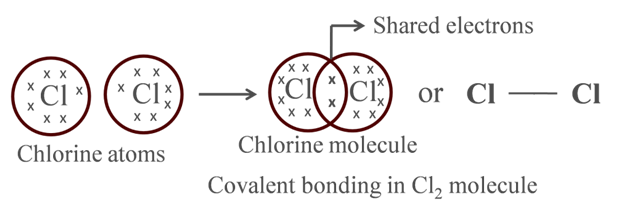
Formation of Chlorine Molecule
Atomic number of Cl = 17
![]()
It has 7 electrons in its outermost shell and thus requires 1 more electron to fulfill its outermost shell.
Formation of Oxygen Molecule
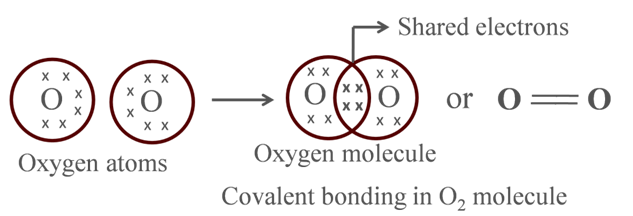
Atomic number of O = 8
![]()
It requires 2 electrons to fulfill its octet for attaining noble gas configuration.
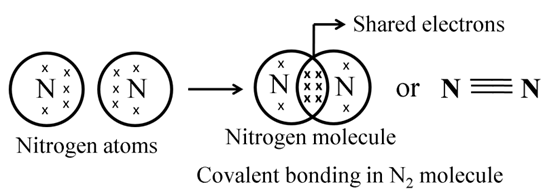
Formation of Nitrogen Molecule
Atomic number of N = 7
![]()
It needs 3 more electrons to attain noble gas configuration.
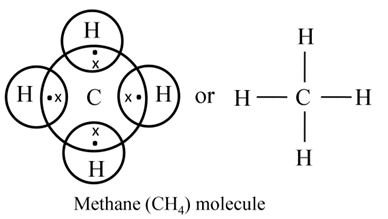
Formation of Methane (CH4)
In the formation of a methane molecule one carbon atom shares its 4 electrons with four different hydrogen atoms
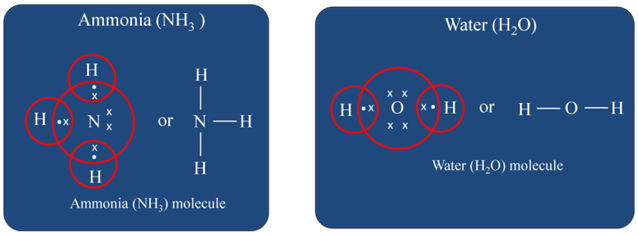
Formation of Ammonia and Water Molecule
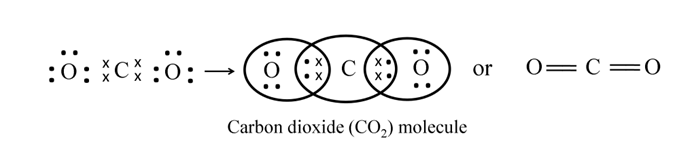
Formation of Carbon Dioxide (CO2)
Atomic number of C = 6
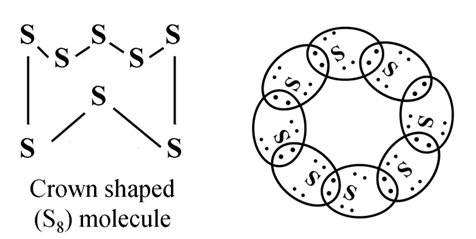
Formation of Sulphur Molecule (S8)
Atomic number of sulphur = 16
Properties of Covalent Compounds
Covalently bonded molecules are called covalent compounds.
- Their constituents are molecules, not ions.
- They have strong bonds within the molecule but intermolecular forces are weak, which is responsible for low melting and boiling points of these compounds (except graphite and diamond).
- In these compounds, electrons are only shared and no charged particle is formed. Therefore, these compounds are bad conductors of electricity due to the absence of free electrons or ions. However, graphite is an exception of it, which is a good conductor of electricity.
- These compounds are generally insoluble in water but some of them are capable to form H bond which are soluble in water.
Difference between Covalent and Ionic Compounds
- Covalent compounds have lower intermolecular force in comparison with ionic compounds.
- Covalent compounds have comparatively lower boiling and melting points than the ionic compounds.
- Covalent compounds are poor conductors of electricity while ionic compounds are good conductors of electricity in their aqueous solution or in molten state.
- Covalent compounds are formed by the sharing of electrons where as ionic compounds are formed by the lose or gain of electrons.
Allotropes of Carbon and Their Properties
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Allotropes of Carbon and Their Properties
Allotropy is the property by virtue of which an element exists in more than one form and each form has different physical properties but identical chemical properties. These different forms are called allotropes.
Carbon exists in two differents allotropic forms
- Crystalline form
e.g. Diamond, graphite and fullerene.
- Non-crystalline form or amorphous form
e.g. Coal, larrrginriark and charcoal.
Diamond General Properties
- It is a colourless transparent substance with extraordinary brilliance due to its high refractive index.
- It is quite heavy.
- It is extremely hard (hardest natural substance known).
- It does not conduct electricity (because of the absence of free electrons).
- It has high thermal conductivity and high melting point.
- It burns on strong heating to form carbon dioxide.
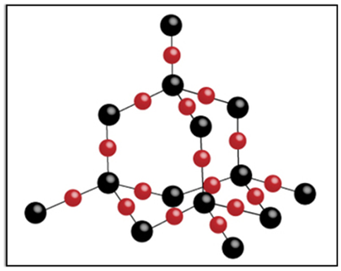
Structure
It is a giant molecule of carbon atoms in which each carbon atom is linked to four other carbon atoms by strong covalent bonds forming a rigid three-dimensional network structure, which is responsible for its hardness.
Uses
- Due to its hardness it is used in knives for cutting marble, granite and glass.
- It is used for the purpose of ornaments studded as precious stones.
- It is used as an abrasive and for polishing hard surface.
- Dies made from diamond are used for drawing wires from the metals.
Graphite General Properties
- It is a grayish black opaque substance.
- It is lighter than diamond, feels soft and slippery to touch.
- Is a good conductor of electricity (due to the presence of free electrons) but bad conductor of heat?
- On strong heating, it burns to give carbon dioxide.
Structure
A graphite crystal consists of layers of carbon atoms or sheets of carbon atoms. Each carbon atom in a graphite layer is joined to three other carbon atoms by strong covalent bonds to form flat hexagonal rings. The various layers of carbon atoms.
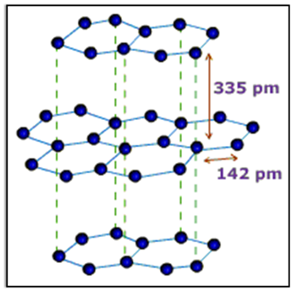
Uses
- Is used as a powdered lubricant for the parts of machinery.
- It is used for making electrodes of cells.
- It is used for making lead for pencils as it can mark paper black. It is therefore called black lead or plumbago.
- It can withstand high temperature so it is used for making crucibles to melt substances with high melting points and tiles on the nose cone of space shuttle.
Fullerenes
These are recently discovered allotropic forms of carbon which were prepared for the first time by H W Kroto, Smalley and Robert Curt by the action of laser beam on the vapours of graphite.
General Properties
- These are dark solids at room temperature.
- These are neither too hard nor too soft.
- These are the purest allotropic forms of carbon because of the absence of free valencies or surface bonds.
- On burning, these produce only carbon dioxide gas. Other properties of fullerene are still being investigated.
Structure
Fullerene (C60) is a football shaped spherical molecule in which 60 C atoms are arranged in interlocking hexagonal and pentagonal rings of carbon atoms.
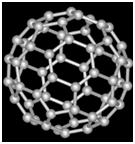
Uses
- In pure form, these behave as insulators. However, these can be converted into semiconductors under suitable conditions.
- C60O, a molecule formed when C60 traps O atoms, is used in cancer as well as AIDS therapy.
- In small amounts, these are used to catalyses the photochemical refining in industry.
Versatile Nature of Carbon
The estimated number of carbon compounds known today is about three million.
The main reasons for this huge number of carbon compounds are
Catenation
The property of self linking of elements mainly carbon atoms through covalent bonds to form long, straight or branched chains and rings of different sizes is called catenation.
Tetravalency of Carbon
Carbon has four electrons in the outermost shell. Hence, its valency is four i.e. it is capable of bonding or pairing with four other carbon atoms or with the atoms of some other monovalent elements like hydrogen, halogen (chlorine, bromine) etc.
Tendency to form Multiple bonds
Due to its small size carbon has a strong tendency to form multiple bonds (double and triple bonds) by sharing more than one electron pair with its own atoms or with the atoms of elements like oxygen, nitrogen, sulphur etc.
Organic Compounds
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Organic Compounds
The compounds of carbon except its oxides, carbonates and hydrogen carbonate salts, are known as organic compounds. These compounds were initially extracted from natural substances.
Hydrocarbons
The organic compounds containing only carbon and hydrogen are called hydrocarbons.
The hydrocarbons can be classified as
- Saturated hydrocarbons
- Unsaturated hydrocarbons
Saturated Hydrocarbons
The hydrocarbons in which all the carbon atoms are connected by only single bonds are called saturated hydrocarbons or alkanes.
The general formula of these compounds is CnH2n + 2
Unsaturated Hydrocarbons
Those carbon compounds in which at least one double or triple bond is present are called unsaturated compounds.
Unsaturated compounds are further divided into following two classes
- Alkenes (CnH2n)
- Alkynes (CnH2n – 2)
Alkenes
Those carbon compounds which have at least one double bond along with single bonds are called alkenes.
General formula of these compounds is CnH2n.
Alkynes
Those unsaturated hydrocarbons which have one or more triple bonds along with the single bonds are called alkynes.
General formula of these compounds is CnH2n – 2.
How to Draw the Structure of Saturated and Unsaturated Compound?
To draw the structure of carbon compound
Step I:- First connect all the carbon atoms by a single bond.
Step II:- After that satisfy the tetravalency of carbon with available hydrogen atom.
Step III:- If number of available H atoms are less than what is required, satisfy the remaining valency by using double or triple bond in between C atoms.
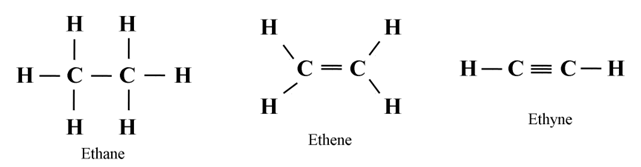
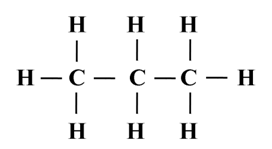
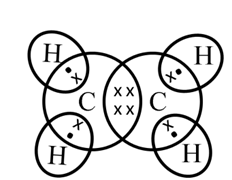
Structure of Ethane (C2H6)
![]()
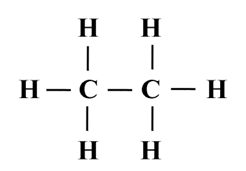
Electron dot structure of ethane (C2H6)
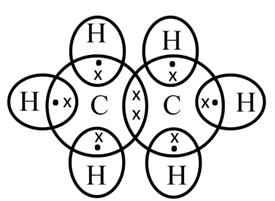
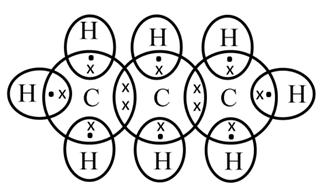
Structure of Propane (C3H8)
Electron dot structure of propane (C3H8)
Structure of Ethene (C2H4)
![]()
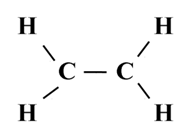
To satisfy it, a double bond is used between the two carbon atoms.
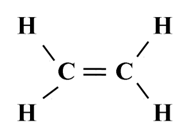
Electron dot structure of ethane
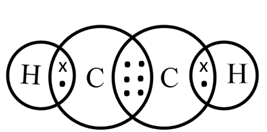
Structure of Ethyne (C2H2)
C ─ C
H ─ C ≡ C ─ H
In ethyne, the two carbon atoms share three pairs of electrons among themselves to form a carbon-carbon triple bond.
Electron dot structure of ethyne (C2H2)
How to Draw Structure of Cyclic Compound?
Some carbon structure also exist in cyclic or ring structure.
To draw the structure of cyclic or ring compound
Step I:- First connect the available carbon atoms by a single bond in the cyclic form.
Step II:- Try to satisfy the tetra valency of each carbon with the available hydrogen atoms.
Step III:- Now check the valency of each carbon. If it is found unsatisfied, use double or triple bond to satisfy it.
For example
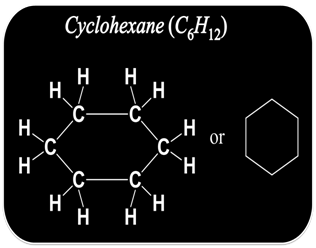
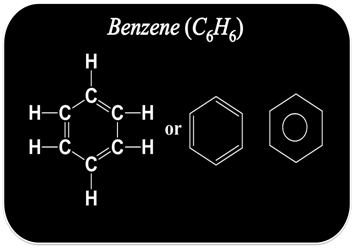
Isomerism
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Isomerism
Carbon compounds or organic compounds with same molecular formula can show different structures and hence, different properties. This phenomenon is called isomerism and compounds are called isomers.
For example
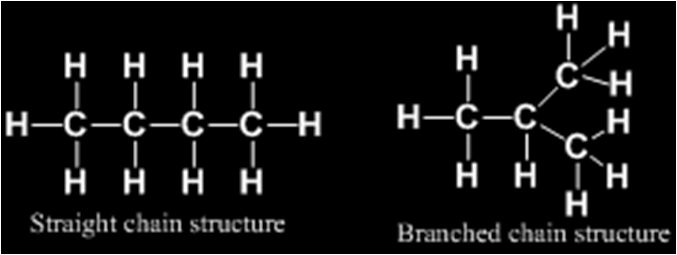
Such pair of isomers is called chain isomers and the isomerism is called chain isomerism.
Molecular formula C5H12
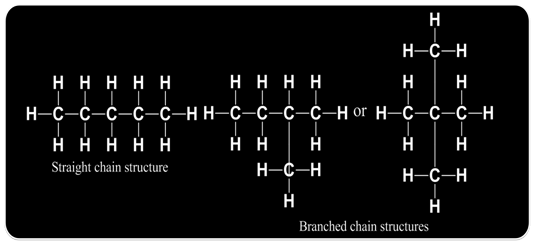
Functional Groups
Functional groups may be defined as an 'atom' or a ‘group of atoms’ which makes a carbon compound reactive and decide its properties regardless of the length and nature of carbon chain.
Homologous Series
A series of similarly constituted compounds in which the members present have the same functional group and similar chemical Properties and any two successive members in a particular series differ in their molecular formula by —CH2— unit, is called a homologous series.
Characteristics of a Homologous Series
- All the members of a homologous series can be represented by the same general formulae.
- Any two adjacent homologues differ by 1 carbon atom and 2 hydrogen atoms in their molecular formulae.
- All the Compounds of a homologous series show similar chemical properties.
- With increase in the molecular mass the gradual change in the physical properties occurs.
- The 14 u is the difference in the molecular masses of any two adjacent homologues:
For example,
CH4, C2H6, C3H8, C4H10 — these all compounds differ by a — CH2 — unit.
Nomenclature of Carbon Compounds
Organic compounds have generally two types of names. These are IUPAC names and common names. The IUPAC names have been adopted by the International Union of Pure and Applied Chemistry. And are based on certain rules. The common names, also known as trivial names, have no proper system for naming.
In general, the IUPAC names of organic compounds are based on the name of basic carbon chain modified by a prefix (phrase before) or suffix (phrase after) showing the name of the functional group.
Writing IUPAC Name of a Compound
Following steps are used to write the name of an organic compound
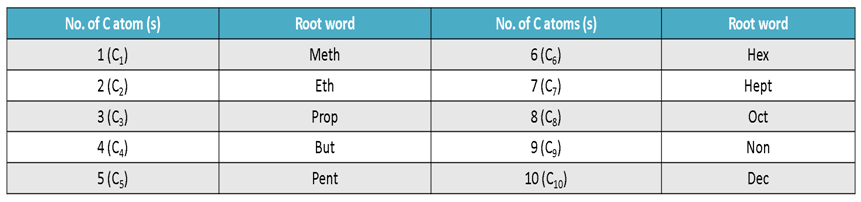
Step I:- Count the number of carbon atoms in the given compound and write the root word for it.
Step II:- If the compounds is saturated, add suffix ‘ane’ to the root word, but if is unsaturated, add suffix ‘ene’ and ‘yne’ for double and triple bonds respectively.
Step III:- If functional group is present in the compounds, it is indicated by adding its suffix.
Root Words for Carbon Atoms
Combustion AND Oxidation
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Combustion And Oxidation
Combustion
All the carbon compounds burn in oxygen and yield carbon dioxide and water vapours. Heat and light are also released during this process. This reaction is called combustion.
For Example
C + O2 → CO2 + Heat + Light
CH4 + 2O2 → CO2 + 2H2O + Heat + Light
2CH3CH2OH + 6O2 → 4CO2 + 6H2O + Heat + Light
Saturated hydrocarbons give a clean flame due to their complete combustion whereas, unsaturated hydrocarbons give a yellow flame with lots of black smoke as they do not undergo complete combustion.
Oxidation
It is the process of intake of oxygen and removal of hydrogen. Reagents used for this purpose are alkaline KMnO4 + heat or acidified K2Cr2O7 + heat.
For Example
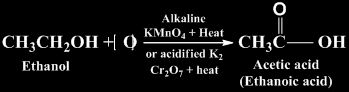
Substances that are capable of providing oxygen to other substances are called oxidising agents.
![]()
Addition Reactions
Addition of hydrogen (hydrogenation) in the presence of catalysts like palladium or nickel, to unsaturated hydrocarbons yields saturated hydrocarbons.
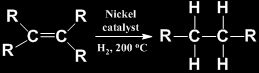
Hydrogenation of vegetable oil in the presence of nickel catalyst gives ghee. This process is called hardening of oils.
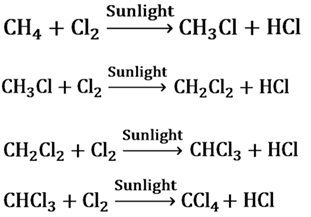
Substitution Reactions
The reactions in which a reagent substitutes an atom or a group of atoms from the reactant are called substitution reaction.
When chlorine is added to hydrocarbons at a rapid rate in the presence of sunlight, Cl replaces H atom one by one.
Some Important Carbon Compounds Ethanol
Its common name is ethyl alcohol and formula is C2H5OH or CH3CH2OH
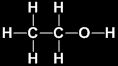
Preparation
Alcohol (ethanol) is obtained by the fermentation of molasses which are obtained from sugarcane juice.
![]()
Physical Properties
Physical properties of ethanol are
- It is a liquid at room temperature. Its melting point is 156 K and boiling point is 351 K.
- It is Soluble in water due to its ability to form H- bonds with water molecules.
Chemical Properties
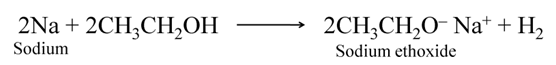
Reaction with Sodium
Ethyl alcohol reacts with sodium metal leading to the evolution of hydrogen gas along with the formation of sodium ethoxide.
Dehydration
Removal of water molecules from a compound is known as dehydration reaction.
For example
When ethanol is heated at 443K with excess conc. H2SO4, the water molecules get removed from it and ethane is obtained.

Uses
Uses of ethanol are
- It is used as an active ingredient in all alcoholic drinks.
- It is useful in medicines like tincture of iodine, cough syrups and may other tonics.
- Alcohol is used as an additive in petrol, since it is a clear fuel and gives rise to only CO2 and H2O when burnt in sufficient air.
- It is used as hypnotic (induces sleep).
- It is used for the preparation of chloroform, iodoform, ethanoic acid, ethanal, etc.
Denatured Alcohol
In order to stop the misuse of ethanol, it is made unfit for drinking by adding poisonous substances like methanol, coppet sulphate, pyridine, etc and coloured substances like dyes. Such alcohol is called. Denatured alcohol.
Ethanoic Acid or Acetic Acid
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Ethanoic Acid or Acetic Acid
It is commonly known as acetic acid. Its formula is CH3COOH. 5-8% solution of ethanoic acid in water is known as vinegar.
Physical Properties
- Its melting point is 290K.
- During winters it often freezes and form ice like flakes, so it is also called glacial acetic acid.
Chemical Reactions
Acidity It is a weaker acid than HCl but stronger than alcohol. It evolves hydrogen when reacts with sodium metal or sodium hydroxide, which shows its acidic nature.
2CH3COOH + 2Na → 2CH3COONa + H2 ↑
CH3COOH + NaOH → CH3COONa + H2O ↑
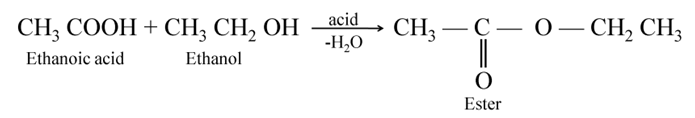
Esterification When ethanol (an alcohol) reacts with acetic acid (a carboxylic acid), a fruity (sweet) smelling liquid called ester is obtained. This reaction is called esterification.
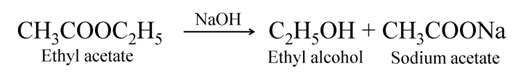
Saponification
The ester gets converted back into alcohol and sodium salt of acid when treated with alkali like sodium hydroxide. This reaction is called saponification, as it is used for the preparation of soap.
Reaction with carbons and hydrogen carbonates in the reaction of acetic acid with carbonates or hydrogen carbonates, carbon dioxide gas is obtained. It is an example of acid-base reaction.
![]()
![]()
Uses of Acetic Acid
- It is used for making vinegar.
- It is widely used as a preservative in pickles.
- It is used for the synthesis of other compounds like esters.
Soaps and Detergents
Soaps are sodium or potassium salts of long chain carboxylic acids and have general formula RCOONa.
Detergents are usually ammonium or sulphonate salts of long chain carboxylic acids.
Manufacture of Soaps and Detergents
Soaps are made from animal fat or vegetable oils by heating it with sodium hydroxide.
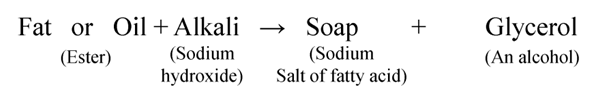
This process of preparation of soap by the hydrolysis of fats and oil with alkalies is called saponification.
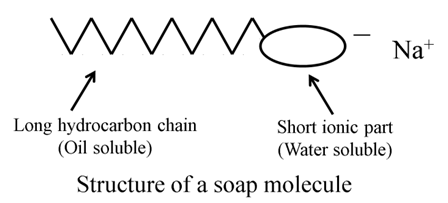
Structure of a Soap Molecule
A soap molecule is made up of two parts — a long hydrocarbon part and a short ionic part containing — COONa group. The long hydrocarbon part is hydrophobic and therefore insoluble in water but soluble in oil. The ionic portion of the soap molecule is hydrophilic so, soluble in water and insoluble in oil.
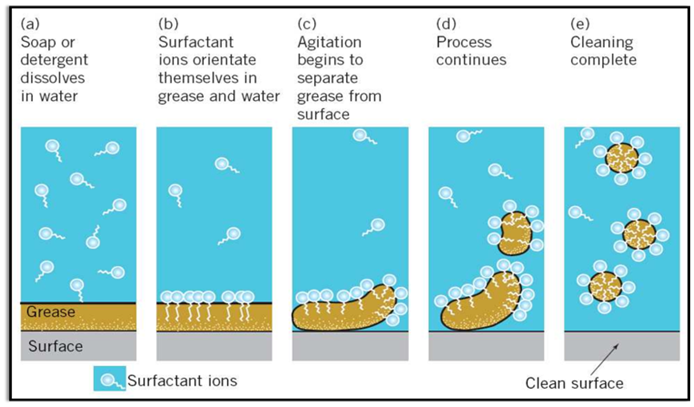
Cleansing Action of Soaps (Micelle Formation)
Some molecules have different properties at their two ends. Its one end is hydrophilic (soluble in water) and other is hydrophobic (soluble in fats or oils).
Inside water these molecules show a unique orientation that keeps the hydrocarbon portion out of the water. This is done by forming clusters of molecules in which the hydrophobic tails are in the interior of the cluster and on the surface of cluster ionic ends are present. This formation is called micelle formation.
Limitations of Soap
Soap is not suitable for washing clothes with hard water. Because hard water contains calcium and magnesium salts. Soap forms scum with hard water as it reacts with calcium and magnesium ions present in the hard water.
Detergents are used as cleansing agents in case of hard water become charged ends of detergents do not form insoluble precipitates with magnesium and calcium ions present in hard water. Hence, they are effective in hard water.
Detergents are useful in making shampoos and products for cleaning clothes but their main disadvantage is that these are generally non-biodegradable.
Advantages of Detergents Over soaps
Synthetic detergents do not require vegetable oil or fats for their preparation, hence they help in saving oils and fats for human consumption.
Synthetic detergents can be used with hard water while soaps cannot be used with hard water.
Periodic Classification of Elements
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Chapter:- 5
Periodic Classification of Elements
Periodic Classification of Elements
Elements are the building blocks of all Substances. There are 118 elements known at present, out of which 98 are naturally occurring. In this chapter, we will study various attempts which have been made to classify the elements from time to time. And finally we will study the modem classification of elements.
Attempts Earlier to Mendeleev
Several attempts have been made to classify elements. We important of which are discussed.
- Lavoisier’s Classification (1789)
- Debereiner's Triads (1817)
- Newlands' Law of Octaves
Lavoisier’s Classification (1789)
It is one of the earliest attempts to classify elements. Lavoisier divided elements into two main divisions, which arc called metals and non-metals.
Debereiner's Triads (1817)
In the year 1817, Johann Wolfgang Dobereiner, a German chemist; identified some groups having three elements each with similar properties. So, he called these groups as triads. When the three elements in a triad were arranged in the order of increasing atomic masses, the atomic mass of the middle element was roughly the average of the atomic masses of the other two elements.
For Example
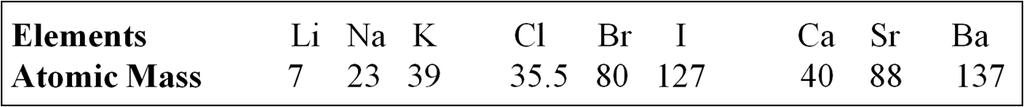
![]()
Newlands' Law of Octaves
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Newlands' Law of Octaves
In 1866. John Newlands, an English scientist; arranged the kown elements in the order of increasing atomic masses' and found that every eighth element had properties similar to that of the first. He called it the law of octaves. This is known as Newland’s law of octaves.
For Example
Newlands’ Octaves
Application
It clearly represented that there was some systematic relationship between the atomic masses and repetition of properties of elements.
Limitations
- This law was successful only up to calcium. After that every eight element did not possess the same properties as those possessed by the elements lying above it in the same group.
- Newlands thought that there were only 56 elements that existed in nature and no more elements would be discovered in the future.
- In order to fit clement into his table, Newlands' adjusted two elements in the same slot, and also put some unlike elements under the same slot.
For Example, cobalt and nickel placed in the same column as fluorine, chlorine and bromine which have very different properties then these elements Iron, which resembles cobalt and nickel in properties, had been placed far away from these elements.
Mendeleev's Periodic Table (1834 – 1907)
Dmitri Ivanovich Mendeleev, a Russian chemist, was the most important contributor for the development of periodic table. 63 elements were known at the Mendeleev's time.
To classify elements
- He examined the relationship between the atomic masses of the elements and their physical and chemical properties.
- Among chemical properties, he concentrated on the compounds formed by the elements with oxygen and hydrogen.
- He took the formulae of the hydrides and oxides formed by an element as one of the basic properties.
Mendeleev's Periodic Law
On this basis Mendeleev formulated a periodic law, which states, “the physical and chemical properties of elements are the periodic function of their atomic masses.”
Features of Mendeleev's Periodic Table
The important features of this table are
- This table contains 8 vertical columns, called groups and 6 horizontal rows, called periods.
- It contains gaps for the elements not discovered at that time. He named such elements by prefixing a Sanskrit numeral eka (one), divi (two), tri (three), etc, to the name of the preceding similar (analogous) clement in the Same group.
For Example eka-boron, eka-silicon, which after their discovery were named as scandium, gallium and getmanium respectively.
- He also predicted the atomic masses and properties of several elements that were not known at that time.
- In order to group the elements having similar properties together, at some places. Mendeleev had to place an element with a slightly greater atomic mass before an element with a slightly lower atomic mass.
- With the help of this table, Mendeleev corrected atomic masses of some elements.
For Example, he corrected the atomic mass of be from 13 to 9.
Limitations of Mendeleev's Periodic Table
This table was greatly helpful for the study of elements but a few anomalies could not be explained on the basis of the table.
These anomalies are
- Position of Hydrogen
- Position of Isotopes
- Uncertainty in Atomic Masses
- Placing of Heavier Element before the Lighter One
Position of Hydrogen
In outer electronic configuration, hydrogen resembles with alkali metals, but tuft like halogen it exists in diatomic form and combines with metals and non-metals to form covalent compounds. Thus, its position was not fixed. But it was kept with alkali metals.
Position of Isotopes
Isotopes are the elements, having similar chemical properties but different atomic masses. In Mendeleev's periodic table, no place was given to these elements"
Uncertainty in Atomic Masses
Another problem was that the atomic mass do not increase in a regular manner in going from one, element to the next. So, it was not possible to predict how many elements could be discovered between two elements especially when we consider the heavier elements.
Placing of Heavier Element before the Lighter One
Few elements, those possess higher atomic mass were placed before elements having a lower atomic mass.
For Example, Argon (39.9) was placed before potassium 39.1, Cobalt (58.9) before nickel (58.6), tellurium (127.60) before iodine (126.9).
Modern Periodic Table and Various Trends in it
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Modern Periodic Table and Various Trends in it
In 1913. Henry Moseley showed that the atomic number of an element is' a more fundamental property and on the basis of this, he modified the Mendeleev's periodic law as “Physical and chemical properties of the elements are a periodic function of their atomic number”. This is called modern periodic law.
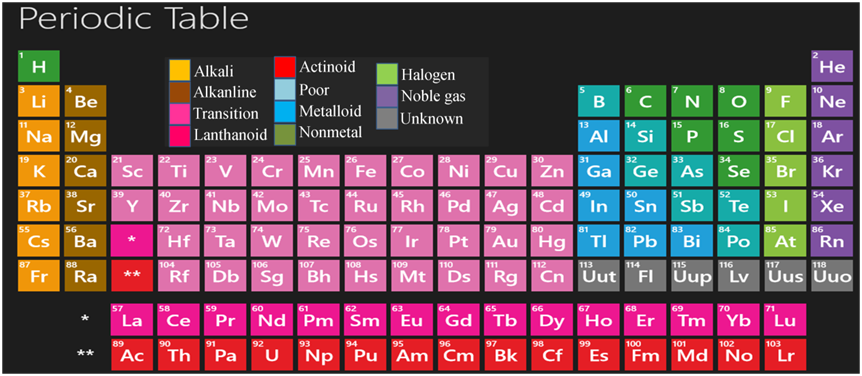
Features of Modern Periodic Table
This table has 18 vertical columns, known as groups and 7 horizontal rows, known as periods.
Features of Groups
- A few important features of the elements present in groups are as follows.
The elements present in a group are separated by definite gaps of atomic numbers.
- The groups are not divided into sub-groups.
- The elements present in a group have the same number of electrons in the valence shell of their atoms or same outermost electronic configuration.
- The elements present in a group have the same valiancy.
- The elements present in a group have identical chemical properties.
- The number of shells increases as we go down the group.
- The elements present in the group 18 are almost chemically uncreative. These are also called noble gas elements.
Features of Periods
The important features of the elements in a period are as follows:-
- Elements of a period do not have the same number of valence electrons but they contain the same number of shells.
- The number of valence shell electrons increase by one unit as the atomic number increases by one unit on moving from left to right in a period.
- As the number of valence shell electrons changes, the chemical properties of the elements also change
- Different periods have different number of electrons which can be explained on the basis of filling of electrons into various shells.
Position of the Elements in the Modern Periodic Table
- In order to find the position of an element in the periodic table, first write its electronic Configuration and then find period and group number as follows.
- The period number of an element is equal to the number of electron shells in its atom.
For Example, if the atom of an element has 2 electron shells (K and L), then it belongs to 2nd period.
- If two (or more) elements have the same number of valence shells, then they belong to the same period of the periodic table.
- The group number of an element having up to two valence electrons is equal to the number of valence electron.
Explanation of the Anomalies of Mendeleev's Periodic Table
Different anomalies of Mendeleev’s table can be explained with the help of modern periodic table as
- The fundamental basis for modern periodic table is atomic number, not atomic mass, hence it is more accurate.
- Since, the table is based on atomic number and isotopes have same atomic number and chemical properties, so they can be put at one place in the same group of the periodic table.
- In this periodic table, a unique, position has been given to the hydrogen. It is kept at the top left corner because of its unique characteristics.
- The position of cobalt and nickel is justified itself because atomic number of cobalt is less than atomic number of nickel.
- A separate group for noble gases was created when these were discovered.
Trends in the Modern Periodic Table
In this table, some properties show a regular trend when we move along a period from left to right or in a group from top to bottom.
These properties are explained in next topics
Valency
It is the combining capacity of an atom of an element to acquire noble gas configuration and depends upon the number of valence electrons the electrons present in the outermost shell.
Variation Along a Group
In a group, outer electronic configuration is same for all the elements, so all have the same number of valence electrons or the valency.
e.g. in case of elements of group 1
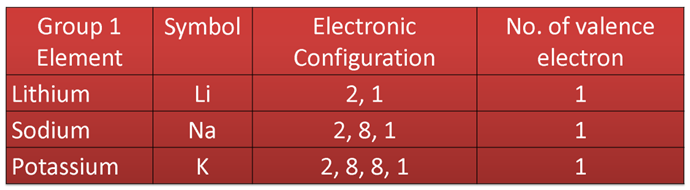
Similarly, for the elements of group 17

Variation Along a Period
Since, the number of valence electrons increases one by one from left to right along a period, so valency also increases from 1 to 7 with respect to oxygen. While with respect to hydrogen it first increases from 1 to 4 and then decreases to 0.

Atomic Size
It refers to the radius of an atom and is defined as the distance between the centre of the nucleus and the outermost shell of an isolated atom.
Variation Along a Period
The atomic radius decreases on moving from left to right along a period. This is due to an increase in nuclear charge which tends to pull the valence electrons closer to the nucleus and reduces the size of the atom
Variation Along a Group
The atomic size increases down the group. This is because new shells are being added as we go down the group. This increases the distance between the outermost electrons and the nucleus.
Metallic and Non-metallic Properties
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Metallic and Non-metallic Properties
Elements, having a tendency to lose one or more electrons and form positive ions are called metals. These are present on the left side as well as in the centre of the periodic table. Because of the formation of positive ions, these arc also called electropositive elements.
Non-metals are the elements which have a tendency to gain one or more electrons to form negative ions. Thus, these are electronegative elements.
There are some elements also which exhibit the properties of both metals and non-metals. These are called metalloids. In the modern periodic table, a zig-zag line separates metals from non-metals. The borderline elements — boron, Silicon, germanium, arsenic, antimony, tellurium and polonium are intermediate in properties so they are called metalloids or semi-metals.
Variation Along a Period and a Group
As the effective nuclear charge acting on the valence shell electrons increases across a period, the tendency to lose electrons will decrease. Down, the group, the effective nuclear charge experienced by valence electrons decreases because the outermost electrons are farther away from the nucleus.
Hence, metallic character decreases across a period and increases down a group Non-metallic character. However increases across a period and decreases down a group.
Nature of Oxides
Oxides of the metals are of basic nature while those of non-metals are acidic. This means that along a period, the basic character of the oxides of the elements decreases while their acidic character increases.
On going down in a group of the periodic table, the order is reverse i.e., basic durum of oxides increases and acidic character of oxides decreases.
Electro Negativity
It may be defined as the relative electron attracting tendency of an atom for a shared electron pair in a covalent bond with other atom.
Variation Along a Period and a Group
The electro negativity of the elements increases along a period since the non-metallic character increases. Similarly, it decreases down the group since the non-metallic character decreases.

 Kaysons Publication
Kaysons Publication
