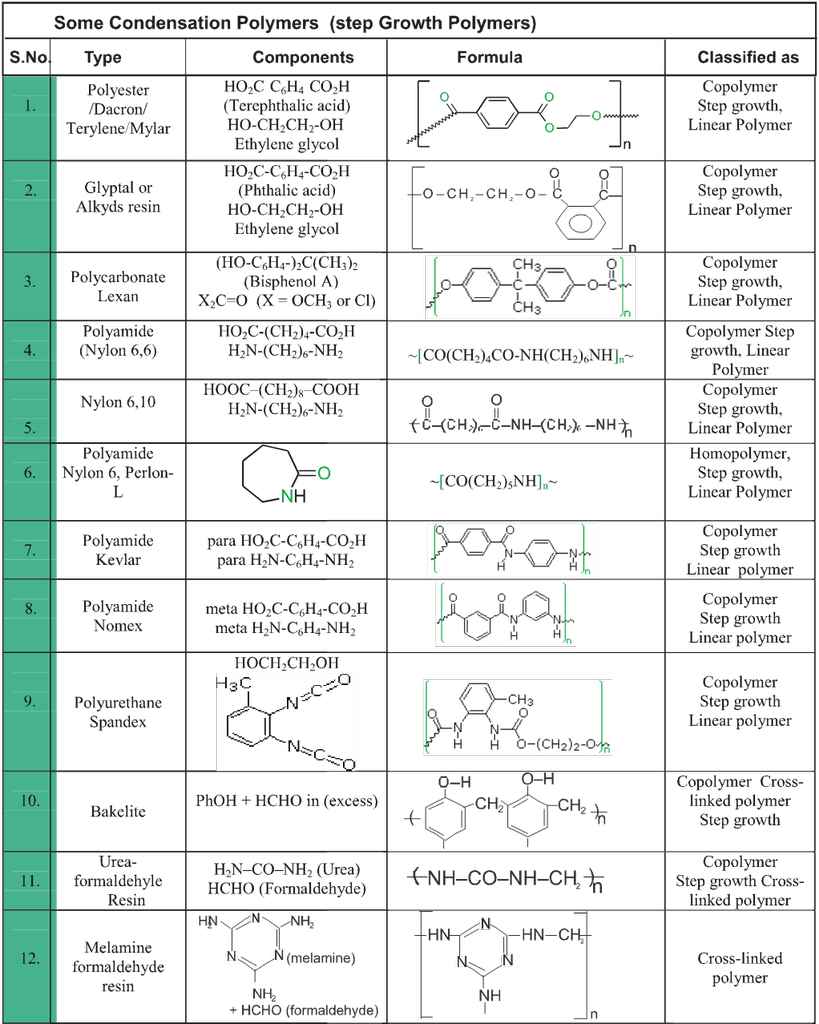
1. Carbohydrates
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Chapter 14: biomolecules & polymers
Carbohydrates (Saccharides) : (Latin word : saccharum = sugar)
Concept
Generally, carbohydrates are substances with the general formula Cx(H2O)y , and were therefore called carboydrates (hydrates of carbon) because they contained hydrogen and oxygen in the same proportion as in water. How ever, a number of compounds have been discovered which are carbohydrates by chemical behaviour but do not conform to the formula Cx(H2O)y e.g ,2-deoxyribose, C5H10O4.
Hence, carbohydrates are biopolymers of polyhydroxy aldehyde or polyhydroxy ketones. “There monomeric polyhydroxy aldehydes or ketones can also exist in hemiocetal and acetal forms in cyclic structures.”
# Note:
Almost all of these compounds are chiral & optically active. “An exception of this is 1,3-dihydroxypropanone, CH2OHCOCH2OH”.
2 Classification of carbohydrates :
(A) Classification on the basis of number of hydrolysed products

(B) On the Basic of functional group
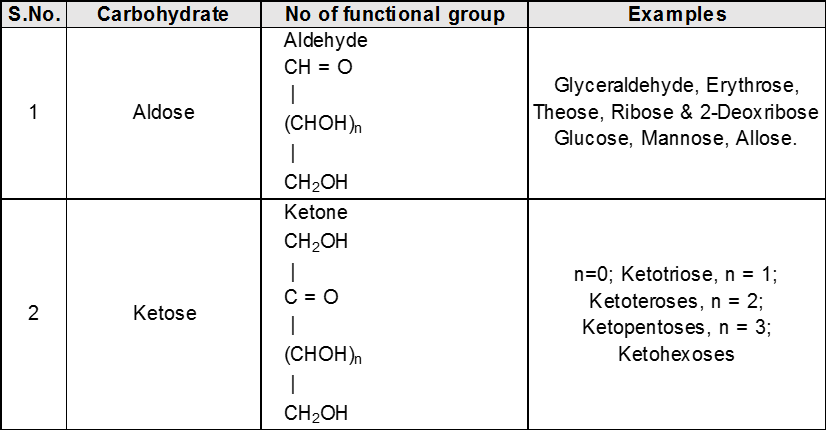
(C) On basis of carbon atoms.

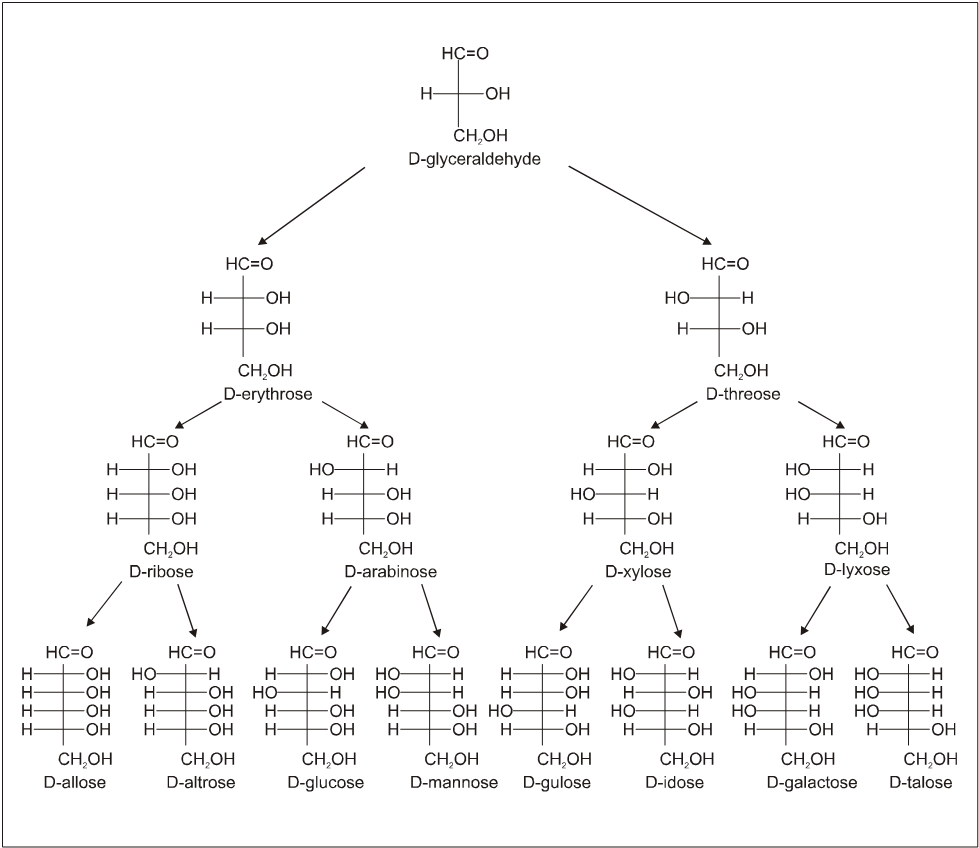
Configuration of the D-Aldoses
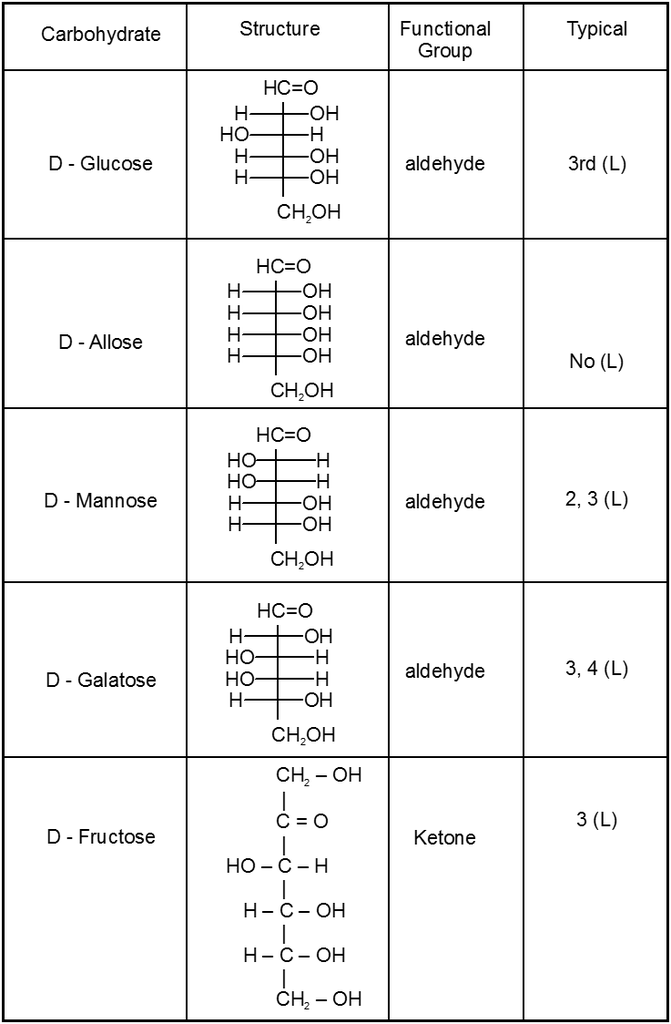
3. Structure :
(i) All form of Aldose or ketose may exist in open chain form as well as in cyclic pyramose/furanose form.
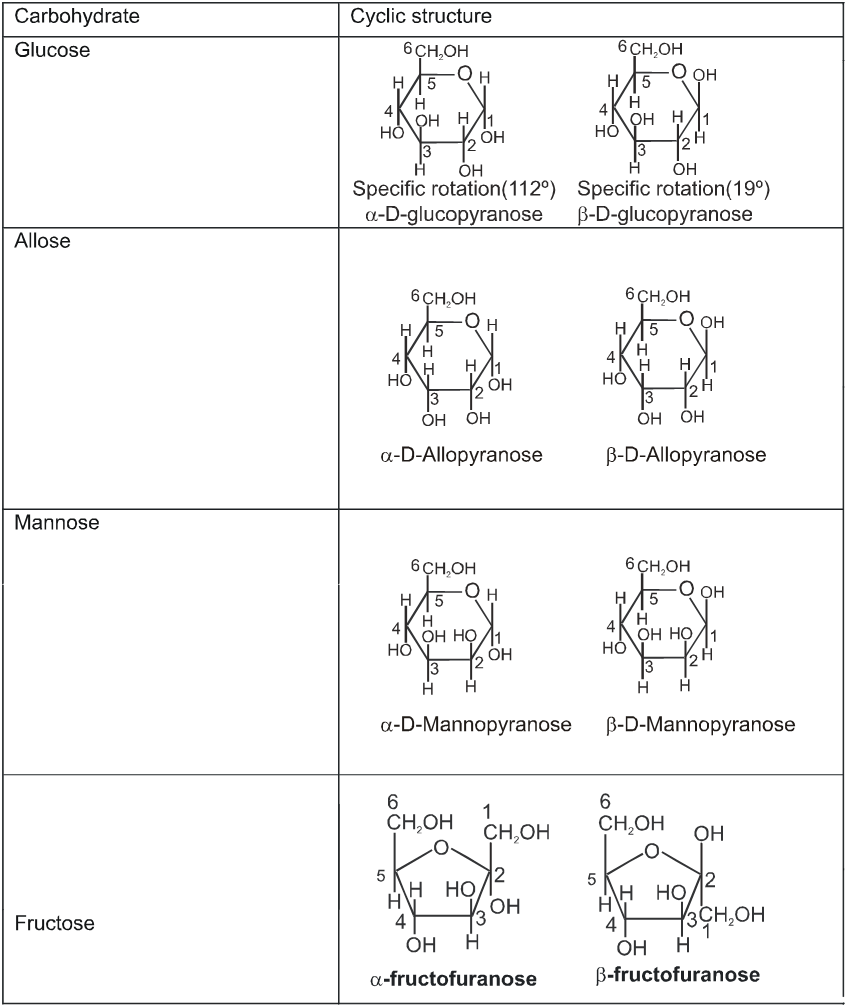
Cyclie structure of monosaccharide
Disaccharide
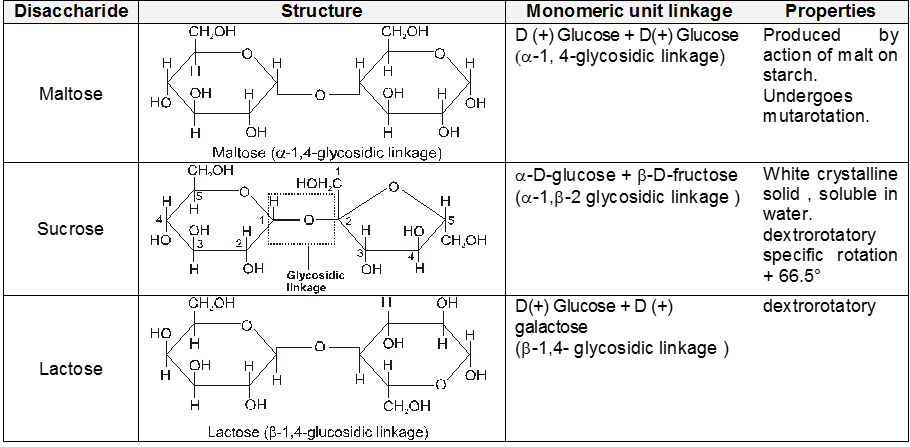
Polysaccharide
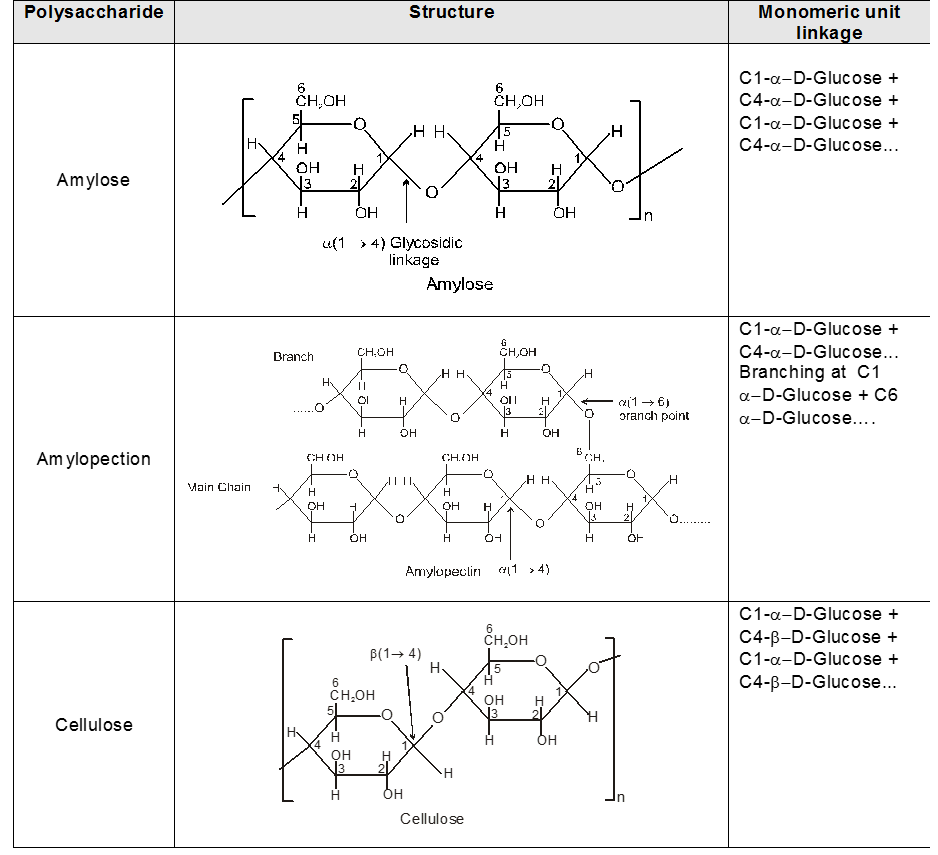
INTER CONVERSION OF a and b
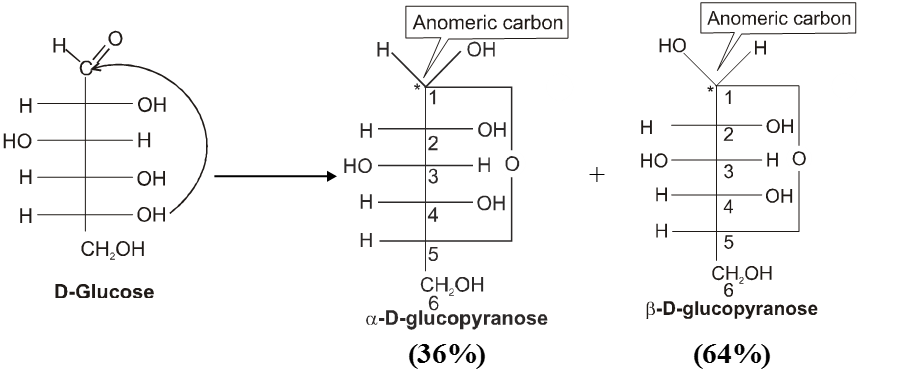
Haworth projection
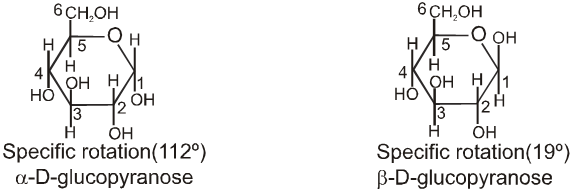
Chair conformation structures :
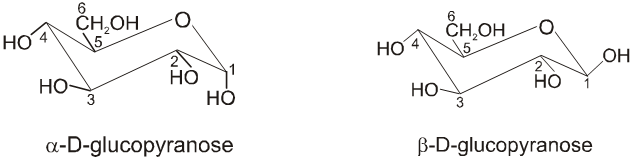
Anomers :
Anomers are diastereomers that differ in the configuration at the acetal or hemiacetal C atom of a sugar in its cyclic form or Anomers are epimers whose conformations differ only about C-1.
For example, a D(+) and b -D(+) glucose are anomers. a-D(–) and b-D(–) fructose are anomers.
Epimers :
Diastereomers with more than one stereocentre that differ in the configuration about only one stereocentre are called epimers.
i. D-Glyceraldehyde and L-glyceraldehyde (this pair is an enantiomer as well as an epimer).
ii. D-Erythrose and L-threose are epimers.
iii. D-glucose and D-galactose are C-4 epimers and
iv. D-idose and D-talose are C-3 epimers.
v. D-glucose and D-mannose are C-2 epimers.
vi. Epimerisation of glucose at C-2 gives mannose.
vii. Epimerisation of glucose at C-3 gives allose.
viii. Epimerisation of glucose at C-4 gives galactose.
Action of alkalies :
(a) With conc. NaOH glucose first turns yellow resinify. Fructose is not effected by conc. NaOH
(b) With dil. NaOH/KOH both Glucose & Fructose undergo a rearrangement to give equilibrium mixture of three sugars.
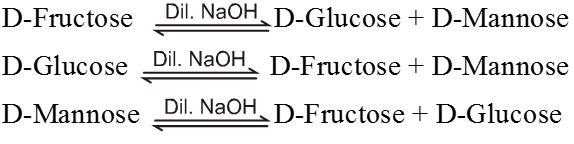
This rearrangement is known as Lobry De-bryn. van Ekenstein rearrangement.
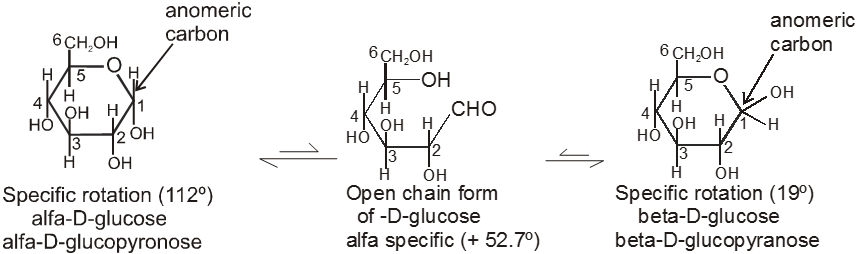
4. Properties of Anomers : Mutarotation
When one of the pure glucose anomers dissolve in water, an intersting change in the specific rotation is observed. When the a-anomer dissolves, its specific rotation gradually decreases from an initial value of +112º to +52.7º. When the pure b- anomer dissolves, its specific rotation gradually increases from +19º to the same value of +52.7º. This change (mutation) in the specific rotation is called mutarotation. What is happening is that each solution, initially containing only one anomeric form, undergoes equilibrium to the same mixture of a-and b-forms. The open chain forms is in intermediate in the process.
For mutarotation atleast one hemiacetal group must be present in the sugar therefore all reducing sugars will mutarotate.
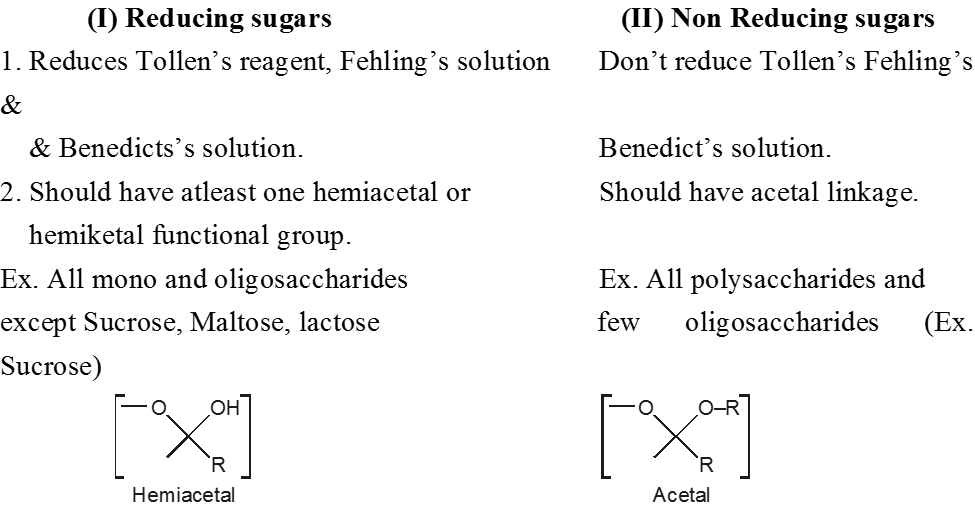
(C) Classification on the basis of Reducing and non Reducing properties :
Monosaccharides :
(A) Glucose (C6H12O6) :
Glucose is the most common monosaccharide. It is known as Dextrose because it occurs in nature principally as the optically active dextrorotatory isomers. It is act as a reducing agent (reduces both Fehling’s solution and ammonical silver nitrate solution). When heated with sodium hydroxide, an aqueous solution of glucose turns brown. It is known as dextrose and found as grapes, honey, cane sugar, starch and cellulose.
Preparation :
(1) By acid hydrolysis of cane sugar (a disaccharide) :
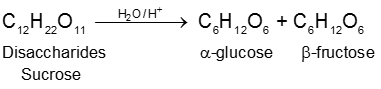
a-Glucose being much less soluble in alcohol than fructose separate out by crystallization on cooling.
(2) By enzymatic action over starch :
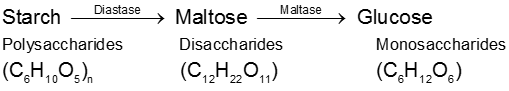
Physical properties :
(i) It is white crystalline solids having melting point 146ºC. It is readily soluble in water.
(ii) Glucose is sweet in taste and also optically active (dextro rotatory).
(iii) Glucose shows mutarotation.
Chemical properties :
(1) Reactions due to OH group :
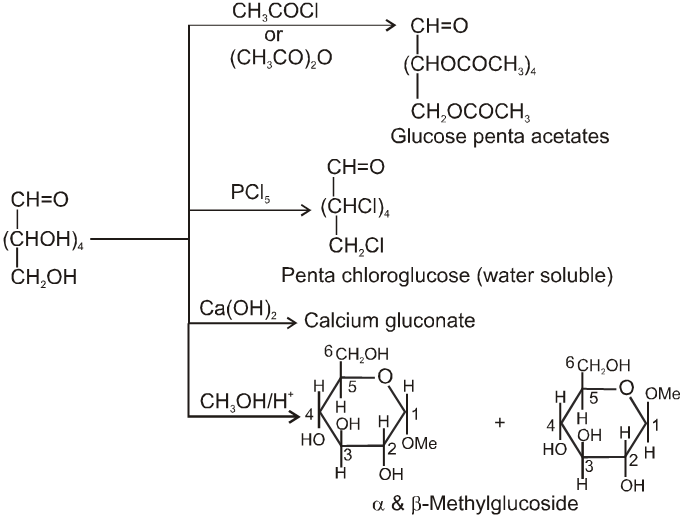
(2) Reactions due to –CHO group :
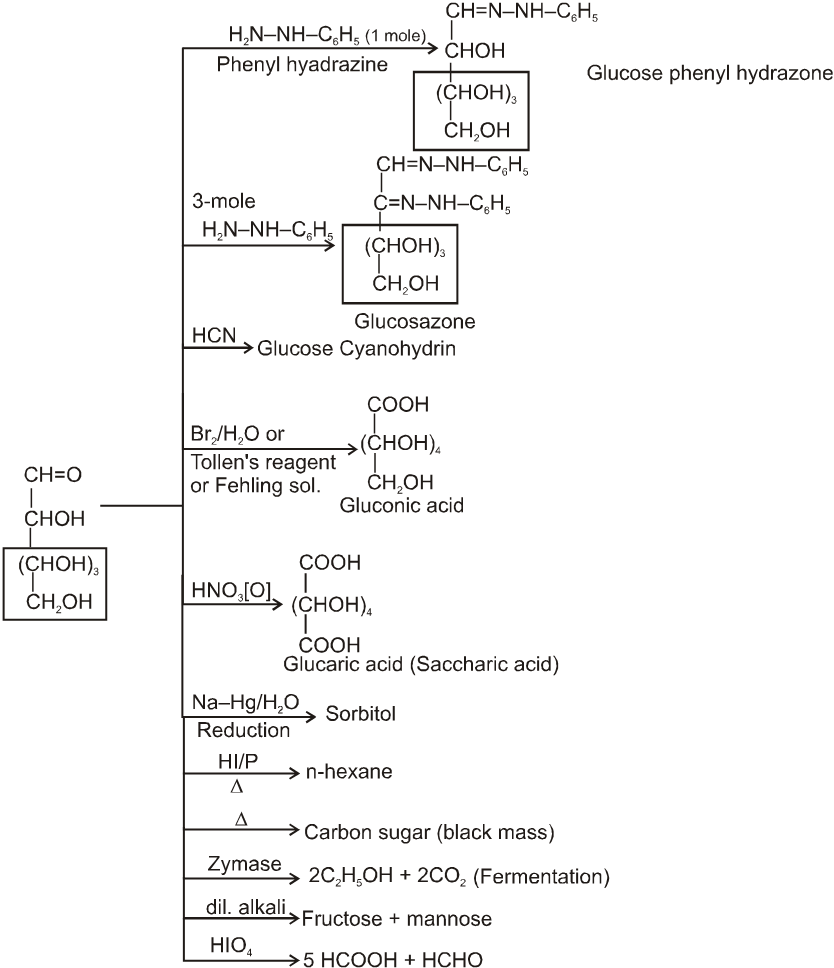
Glycosides-formation :
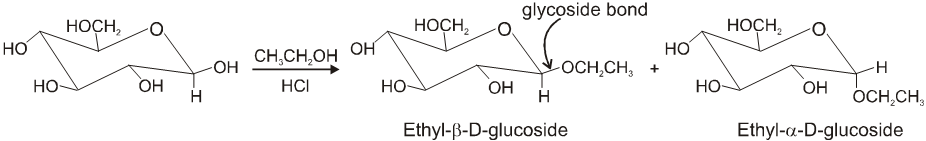
(B) Fructose :
- Also said to be fruit sugar
- It occur both in combined as well as free state.
- Fructose is named as fruit sugar because it is present in honey and most sweet fruit in free state.
- It is sweetest monosaccharide and present in cane sugar and insulin in combined state.
- It is also known as a-Laevulose i.e. natural occurring compound is laevorotatory
Preparation :
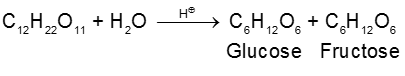
By acid hydrolysis of cane sugar :
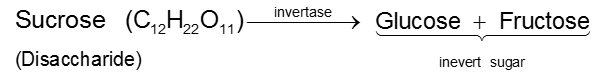
By enzymatic action of sucrose
Note: Glucose and fructose obtained by acid hydrolysis of sucrose can be separated by treating with Ca(OH)2 which forms calcium glucosate & calcium fructosate. Calcium fructosate being water insoluble. It is seperated out easily
Physical properties :
(1) It is colourless crystalline solid.
(2) It is soluble in water but insoluble in ether ketone and benzene.
(3) It is pentahydroxy ketone and shows mutarotation like glucose.
(4) It is reducing sugar.
Chemical Properties :
Due to OH group:
(1) It froms fructose penta acetate with acetyl chloride :
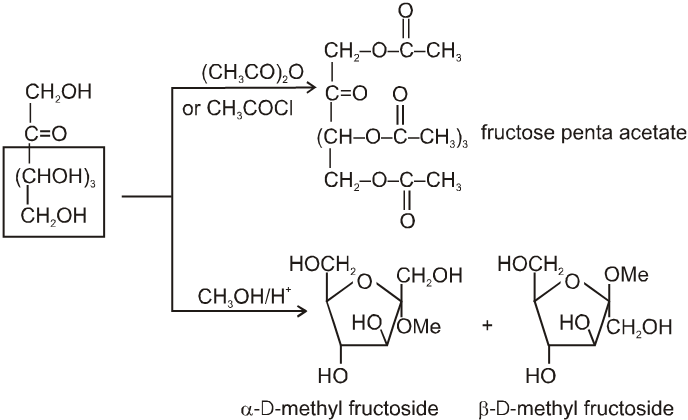
(2) Reaction due to keto group :
It also form osazone with excess of phenyl hydrazine thus we can say that osazone formation is characteristic of a-hydroxy carbonyl compounds.
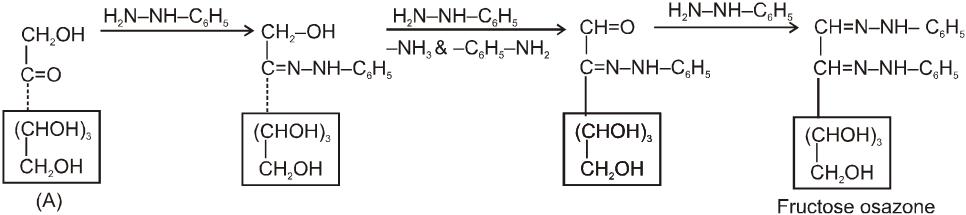
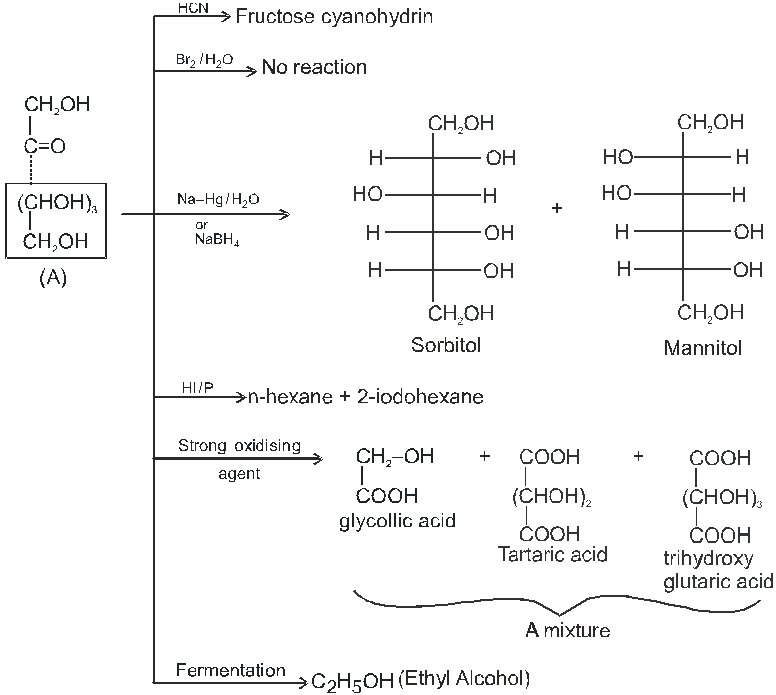
Note : 1. Unlike other ketones, fructose can reduced Fehling sol. and Tollen’s reagents it is probably due to formation of an equilibrium between glucose, mannose and fructose.
Chain lengthing :
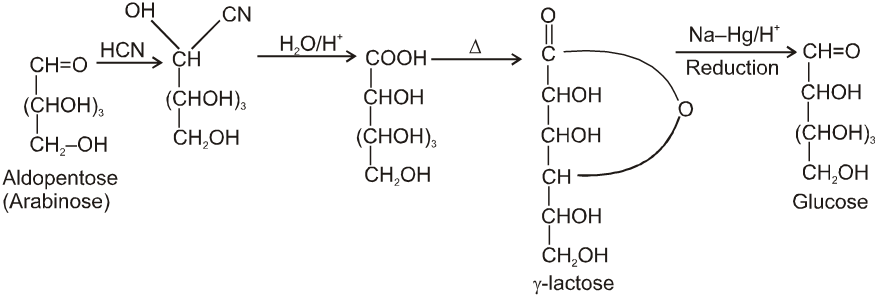
Chain shortening :
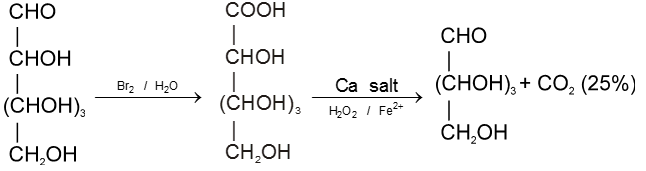
Disaccharides :-
(A) Sucrose : (Sucrose, Invert-sugar C12H22O11)
(a) Sucrose (Cane sugar) ![]() a–glucose + b-fructose
a–glucose + b-fructose
Formation of sucrose (C12H22O11)
a–D-glucose + b-D-fructose
C1 C2
Pyranose form Furanose form
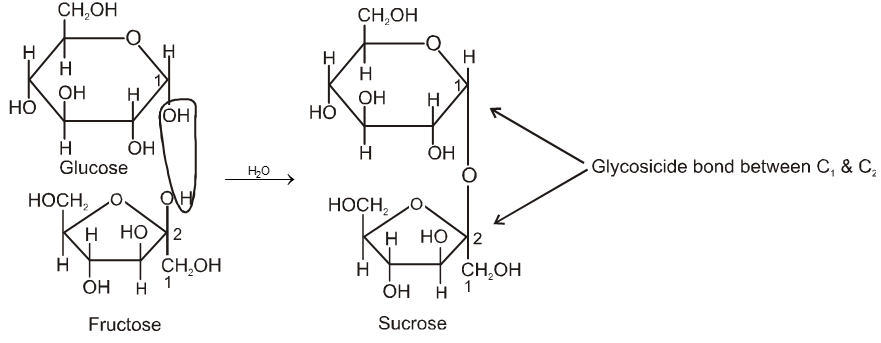
Two monosaccharides are joined together by an oxide linkage formed by loss of water molecule. Such linkage through oxygen atom is called glycosidic linkage.
In sucrose linkage in between C1 of a-glucose and C2 of b-fructose. Since the reducing group of glucose & fructose are involved in glycosidic bond formation, sucrose is non reducing sugar.
(i) On hydrolysis with dilute acids sucrose yields an equimolecular mixture of D(+)-glucose and
D(–)-fructose :
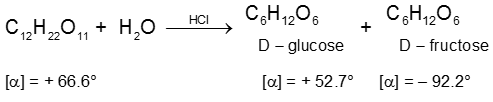
Since D(–)-fructose has a greater specific rotation than D(+)-glucose, the resulting mixture is laevorotatory. Because of this, hydrolysis of cane-sugar is known as the inversion of cane-sugar or Inversion of sucrose (this is not to be confused with the Walden inversion), and the mixture of sugars are known as invert sugar Ex. D - Glucose & D-Fructose. The inversion (i.e., hydrolysis) of cane-sugar may also be effected by the enzyme invertase which is found in yeast.
(ii) Sucrose is not a reducing sugar, e.g., it will not reduce Fehling’s solution or Tollen’s reagnet. It does not form an oxime or an osazone, and does not undergo mutarotation. This indicates that hemiacetal group is not present in the rings.
Polysaccharides :-
(A) Starch (C6H10O5)n :
(i) Starch is the main contributor of carbohydrates in our diet. It exists exclusively in plants, stored in the seeds, roots, and fibres as food reserve. Example rice, potato.
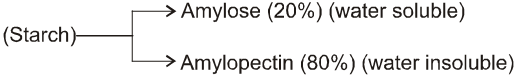
(ii) Both amylose and amylopectin are composed of D-glucose units.
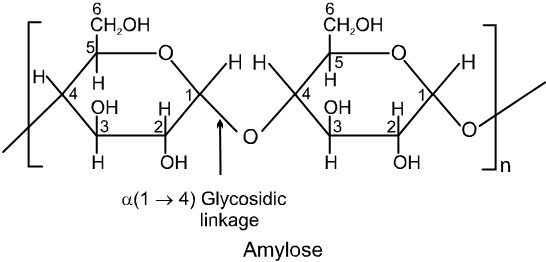
(iii) The amylose molecule is made up of D-glucose unit joined by a-glycosidic linkages between C-1 of one glucose unit and C-4 of the next glucose unit. The number of D-glucose units in amylose range from 60-300.
(iv) Amylopectin has a branched-chain structure. It is composed of chains of 25 to 30 D-glucose units joined by a-glycosidic linkages between C-1 to one glucose unit and C-4 of the next glucose unit. These chains are in turn connected to each other by 1, 6-linkages.
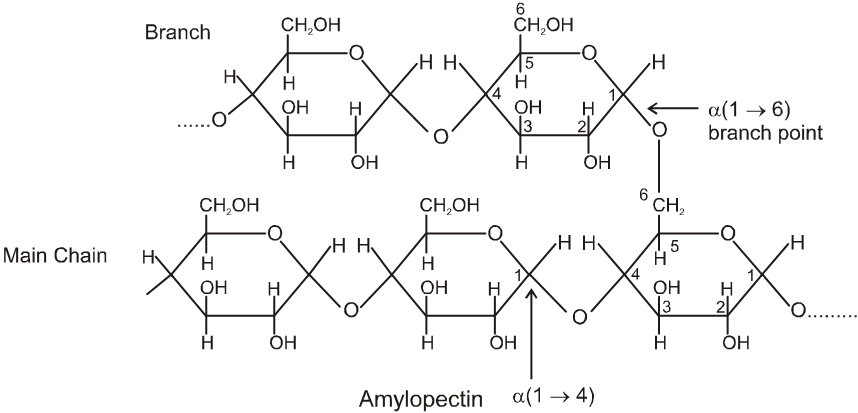
The solution of a-amylose gives a blue colour with iodine.
The solution of Amylopectin gives a violet colour with iodine :
(B) Cellulose, (C6H10O5)n :
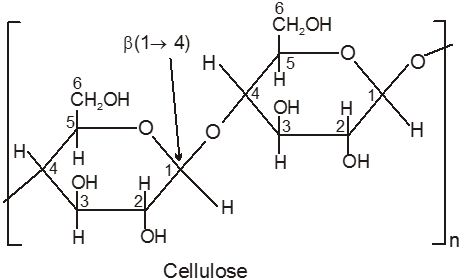
(i) Cellulose is the main structural material of trees and other plants. Wood is 50% cellulose, while cotton and wool are almost pure cellulose. Other sources of cellulose are straw, corncobs, bagasse, and similar agricultural wastes.
(ii) Artificial silk, rayon, is used collectively to cover all synthetic or manufactured fibres from cellulose.
(iii) The nitrates are prepared by the reaction of cellulose with a mixture of nitric and sulphuric acids, and the degree of ‘nitration’ depends on the concentrations of the acids and the time of the reaction. Cellulose trinitrate (12.2 – 13.2%N) is known as gun-cotton and is used in the manufacture of blasting explosives and smokeless powders.
(iv) Non-reducing sugar.
2. Proteins
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Proteins
Proteins : Proteins are the most abundant-biomolecules of the living beings. The chief source of proteins are milk, cheese, pulses peanuts, fish meat etc. These are high molecular mass complex, biopolymers of amino acids. The word protein is derived from Greek word proteios which means primary or of prime importance.
Amino Acids : Each living cell is made up of thousands of different proteins All proteins contain the elements like carbon, hydrogen, oxygen, nitrogen and sulphur. Some of these also contain phosphorus, iodine and traces of metals such as, Fe, Cu, Zn, & Mn. All proteins are polymers of a-amino acids and on partial hydrolysis give peptides of varying molecular masses which upon complete hydrolysis give a-amino acids.
![]()
Type of a-amino acids :
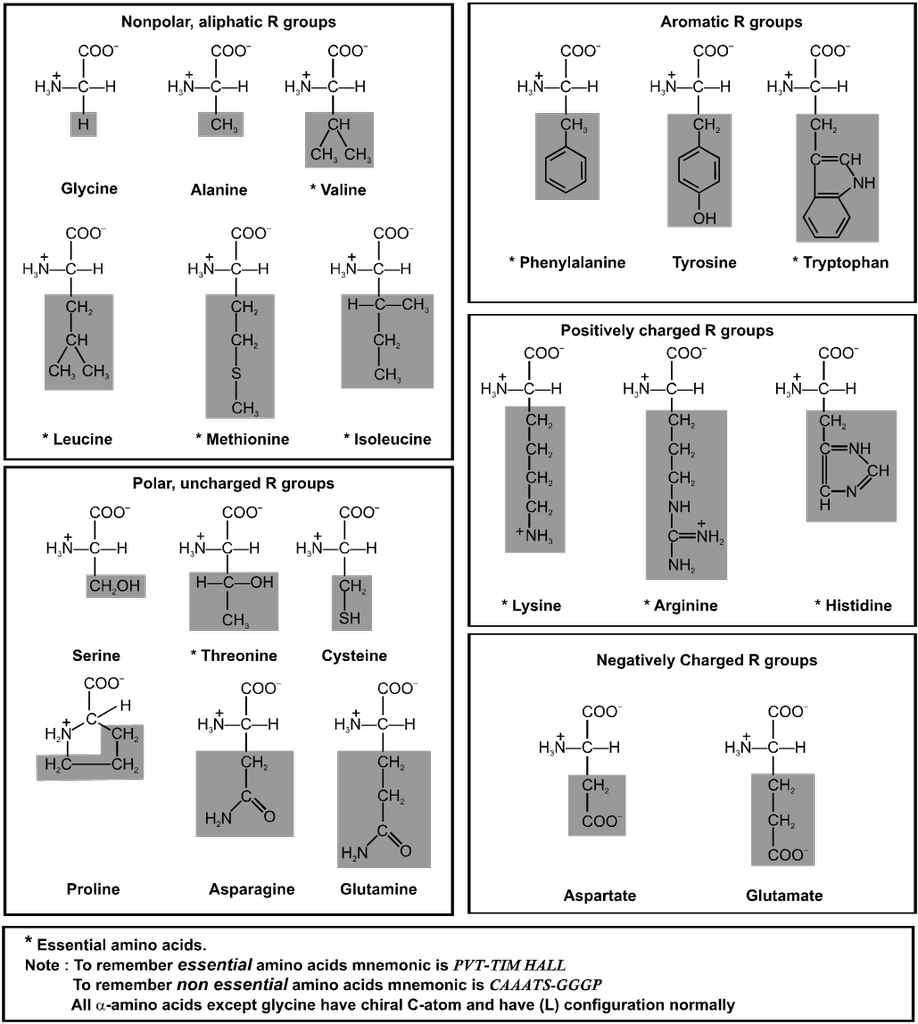
These organic compounds contain amino as well as carboxylic acid group. On the basis of position of amino group in the chain, these are named as a, b, g etc. amino acid.
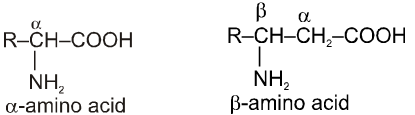
Amino Acid : Building Blocks of Proteins
Amino acids are the building blocks of the molecular structure of the important and very complex class of compounds known as proteins. The proteins on hydrolysis yield mixture of the component amino acids.
Amino acids are bifunctional compounds containing both an amino and a carboxylic acid group. They are represented by the general formula :
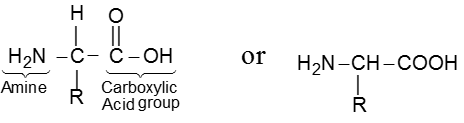
where, R = alkyl, aryl, or any other group.
Zwitter ion (Dipolar Nature of Amino acids) :
In a neutral amino acid solution, the –COOH loses a proton and the –of the same molecule picks up one.The resulting ion is dipolar, charged but overall electrically neutral. This is called Zwitterion (German, “two ions”). Therefore amino acids are amphoteric.
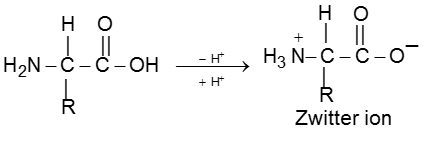
There are around 20 amino acids in the living system.
Classification of Amino Acids :
(A) On the basis of synthesis :
(i) Non essential amino acids : 10 amino acids are synthesis in our body and these are said to be non essential amino acids (eg. Gly, Ala, Glu, Asp, Pro and Cys).
(ii) Essential amino acid : 10 amino acids which are necessarily be present in our diet are called essential amino acids (eg. Val, Leu, I leu, Lys and Phe).
(B) On the basis of functional groups present :
(i) Neutral amino acids : If only one – NH2 and one – COOH groups are present. eg. Glycine, Alanine, Valine, Leucine etc.
(ii) Acidic amino acid : If one basic and two acidic groups are present. Additional acidic functional group must be present in the side chain. E.g. Aspartic acid and Glutamic acid.
(iii) Base amino acid : If two basic and one acidic group is present. Additional basic group must be present in the side chain. E.g. Arginine, Lysine & Histidine.
Isoelectric point of a-amino acids :
When an ionised amino acid is placed in an electric field, it will actually migrate towards the opposite electrode. Depending upon the pH of the medium, three things can happen. The positive form (II) will migrate to the cathode, the neutral form (I) (Zwitter ion) will not migrate, while the negative form (III) will migrate to the anode. The pH at which the amino acid shows no tendency to migrate when placed in an electric field is known as its isoelectric point. This is characteristic of a given amino acid. Thus glycine has its isoelectric point at pH 6.1.
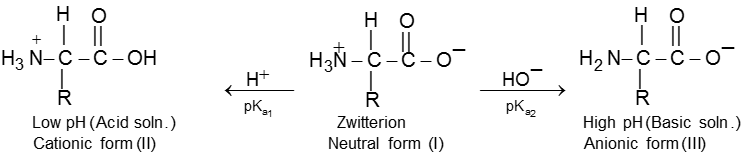
Isoelectric Point : The pH at which the amino acid shows no tendency to migrate when placed in an electric field is known as its isoelectric point.
Because of amphoteric nature in acidic solution it exist as the +ve ion. Hence it migrate towards cathode while in basic solution it exist as –ve ion and migrates towards anode.
In strongly acidic medium
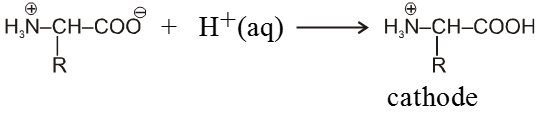
In strongly basic medium
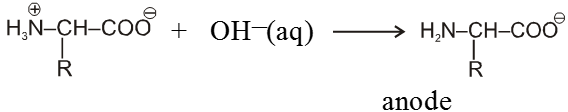
At some intermediate pH amino acids exist as a neutral dipolar ion i.e. the concentration of the cation and anions are equal and it does not migrate towards either electrode, this pH is called iso electric point of amino acid which is different for different amino acids.
For example :
(i) For neutral amino acid : pH of isoelectric point varies between 5.1 to 6.5. e.g. Glycine has pH value 6.0.
PI for neutral amino acid is calculated as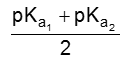
(ii) For acidic amino acid : Where there are two COOH groups and one NH2 group then isoelectric pH is around 3. e.g. Aspartic acid.
Aspartic acid :
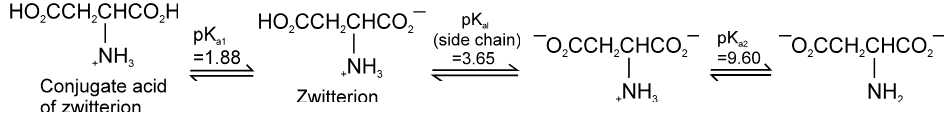
PI for acidic amino acid is calculated as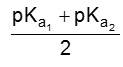
The pI of aspartic acid is the average of pKa1 (1.88) and the pKa of the side chain (3.65) or 2.77.
(iii) For basic amino acid : where there are two NH2 groups and one COOH group then isoelectric point varies between 7.6 to 10.8.
e.g. Lysine (9.8)
Lysine :
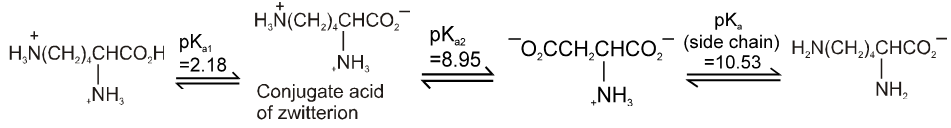
PI for basic amino acid is calculated as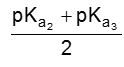
The PI of lysine is the average of pKa2 (8.95) and the pKa of the side chain (10.53) or 9.74.
Note : Amino acid has minimum aqueous solubilities at their isoelectric points.
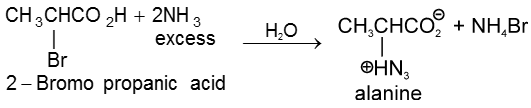
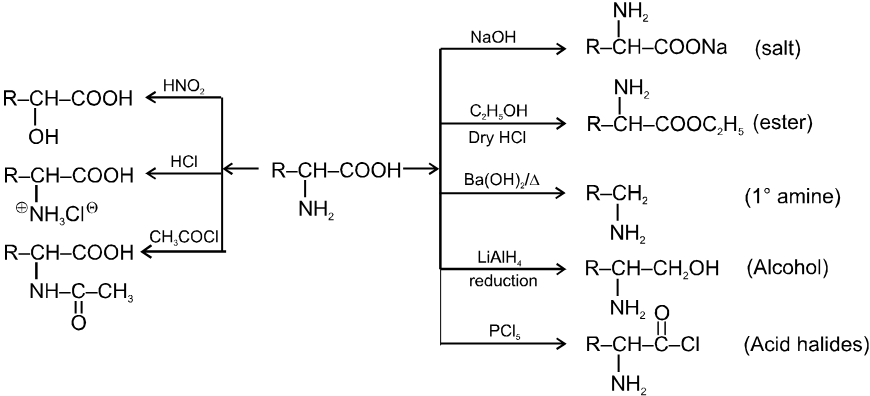
(a) General methods of preparation
1. Aminolysis of a-halocarboxylic acid
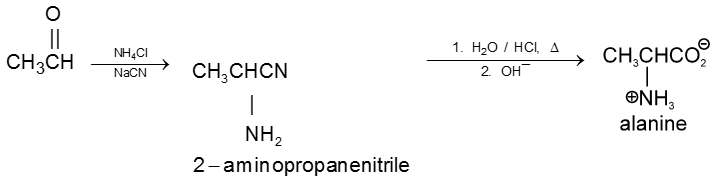
2. By strecker synthesis : Aldehyde reacts with a mixture of NH4Cl and NaCN to form a-aminonitrile (as an intermediate) which on hydrolysis gives an amino carboxylic acid.
3. From aceto acetic ester :
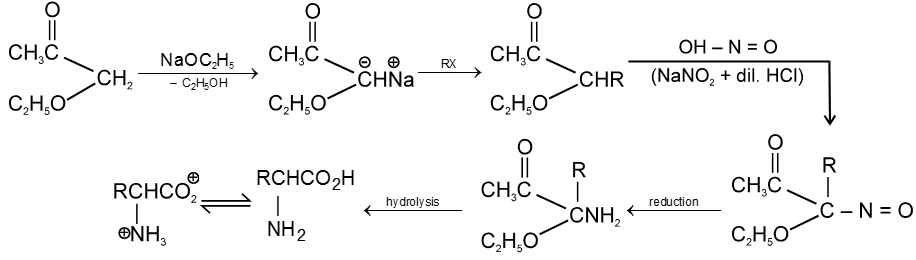
4. By Gabriel Synthesis :

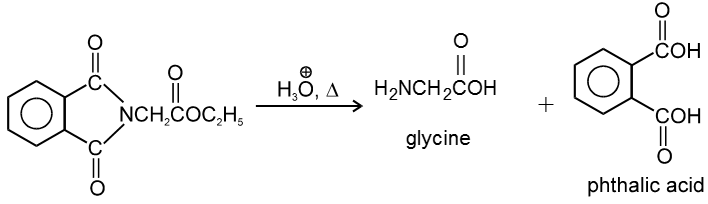
5. Effect of Heat :
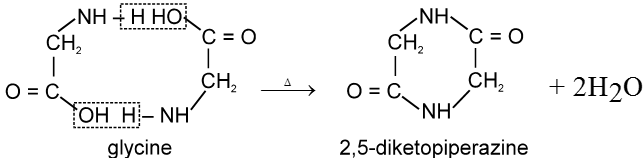
a-amino acids undergo intermolecular dehydration on heating at about 200°C to give diketopiperazines.
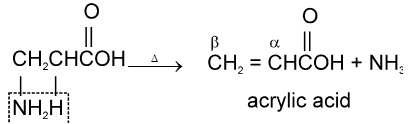
b-amino acids undergo intramolecular deamination on heating to form a, b-unsaturated acids.
g-amino acids and d-amino acid undergo intramolecular dehyderation to form cyclic amides called.
Lactams.
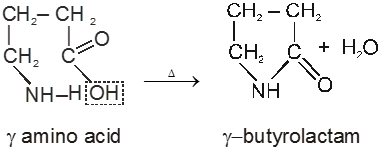
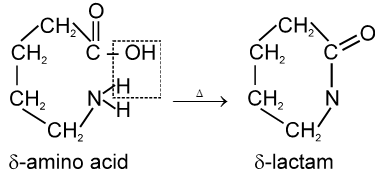
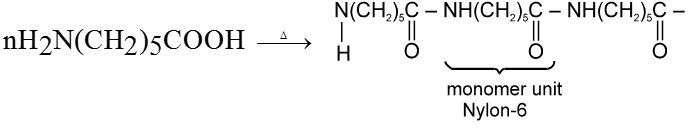
In case of d-amino acid, intramolecular cyclisation would given a seven-membered ring, which is formed with difficulty. Hence, there is intermolecular polymerisation forming nylon-6.
(b) Other Reactions of a-Amino Acid :
Peptide linkage :
Amino acid units are linked together by peptide ![]() – linkage and form polypeptides.
– linkage and form polypeptides.
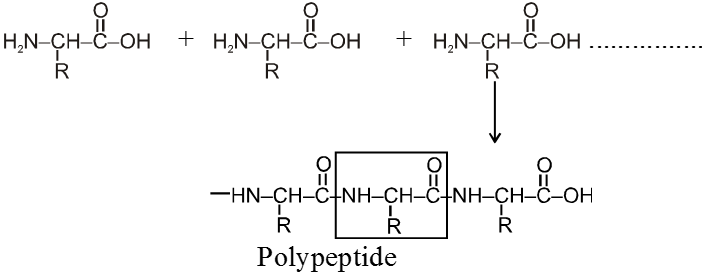
Note : Formation of protein linking together from different amino acids is not a random process. Each molecule of a given protein have the same sequence of amino acid along with its polypeptide chain. This tells about its own specific properties. Even the change of just one amino acid can change drastically the properties of entire protein molecules.
e.g. hemoglobin has 574 amino acids in definite sequence. The change of only one amino acid makes it defective hemoglobin which looses its property of carrying oxygen in blood stream and result a disease called sickel cell animea.
Writing and Naming of Polypeptides :
The structure of polypeptides are written in such a way that amino acid with free –NH2 group is written on the left hand side (N-terminal) of the polypeptide chain, while the amino acid with the free carboxyl group is written on the right hand side (C-terminal) of the chain. Thus a tripeptide alanylglycylphenylalanine (Al, Gly, Phe) is represented as follows :
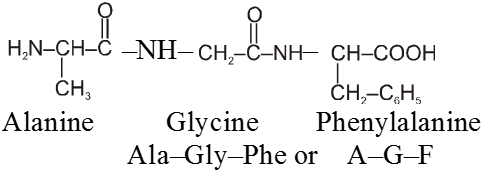
The name of any polypeptide is written starting from the N–terminal residue while writing the name the suffix “ine” in the name of the amino acid is replaced by “yl” (eg. glycyl for glycine, alanyl for alanine etc) for all the constituent amino acid except the C– terminal residue. This nomenclature is usually not used. Instead the three letter abbreviation or one letter codes for various a-amino acids present in the chain is used. For example the above tripeptide is named as Ala-Gly-Phe or A-G-F
Properties of polypeptides :-
(i) Polypeptides are amphoteric in character because of the presence of terminal ammonium and carboxylate ion as well as the ionised side chain of amino acid residues. Therefore like amino acids they neutralize both acids and bases and possess isoelectric points. At isoelectric point polypeptides have least solubility and hence can be separated.
(ii) The total contents of smaller peptides in tissues is small as compared to protein. However, like protein they also carry out many important functions in bio-system. Most of the toxins (poisonous substances) in animal and in plant sources are polypeptides. Some oligopeptides such as oxytoxin, vassopressin and angiotens are effective hormones while others act as sweetening agents. For example the dipeptide aspartyl phenyl alanine methyl ester called aspartame is 100-200 times more sweet than sucrose and is used as a suger substitute for dibetic patients
 Aspartame
Aspartame
Proteins :
Proteins are the most abundant-biomolecules of the living beings. The chief source of proteins are milk, cheese, pulses peanuts, fish meat etc. These are high molecular mass complex, biopolymers of amino acids. They occur in every part of the body and form fundamental basis of structures and determine functions of life. The word protein is derived from Greek word proteios which means primary or of prime importance.
Proteins are present in almost all the living cells of plant and animals. The protoplasm of plant and animal cells contains (10-20%) proteins. In human beings they are the main constituent of muscles, skin, hair, nail, tendons, arteries and connective tissue.
Classification of Proteins :
(I) On the basis of molecular structure :
(i) Fibrous proteins
(ii) Globular proteins
(i) Fibrous Proteins : When polypeptide chain run parallel and are held together by hydrogen and disulphide bonds then fiber like structure is formed such proteins are generally insoluble in water.
Ex. Keratin, myosine, collagen, etc.
(ii) Globular proteins : The polypeplide chain in these proteins is folded around it self to form spheroidal shape. The folding takes place in such a manner that lipophilic (non-polar, fat soluble) parts are pushed inwards and hydrophilic part (polar, water soluble) are pushed outwards. As a result water molecules interact strongly with the polar groups and hence globular proteins are soluble in water. As compare to fibrous proteins these are very sensitive to small changes in temperature and pH.
e.g. Insulin, albumin, thyoglobulin, antiadiodies, haemoglobin fibrinogen etc.
(II) On the basis of chemical composition :
(i) Simple proteins : These proteins on hydrolysis give only a-aminoacids. e.g. albumin in the white portion of eggs, glutenin in wheat, oxygenin in rice, keratin in hair, nails, horns etc.
(ii) Conjugated Proteins : These are the proteins in which protein part is combined with non-protein part. These proteins on hydrolysis give a non-protein in addition to the a-amino acids (Prosthetic group). The main function of prosthetic group is to control the biological function of the protein. Prosthetic groups are carbohydrate, phosphate group, lipids (ester of higher fatty acids) and so on. Casein of milk, haemoglobin of blood are conjugated proteins.
(iii) Derived Proteins : These are the degradation products obtained by partial hydrolysis of simple or conjugated proteins with acids, alkalies or enzymes. e.g. proteoses peptones, and polypeptides
Protein ® Proteoses ® Peptones ® Polypeptides
Structure of Proteins :
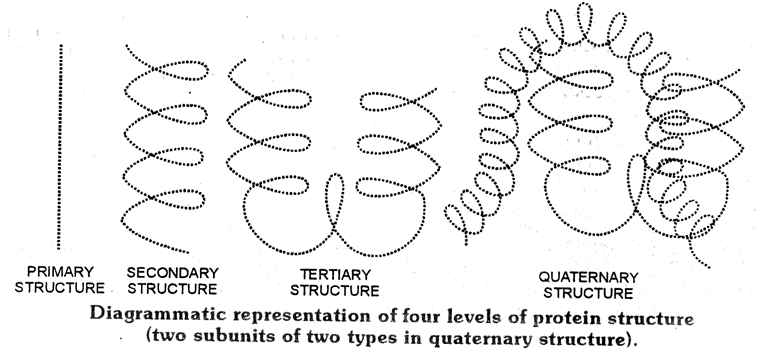
Proteins are biopolymers having a large number of amino acids through peptide bonds having three dimensional (3D) structures. Structure and shape of proteins can be studied and evaluated at four different levels i.e. primary, secondary, tertiary and quarternary, each level being more complex than the previous one.
(i) Primary structure of Proteins :
The manner and specific sequence in which different amino acids are joined to form polypeptides in a protein molecule is called 10 structure of that protein. Any change in this primary structure i.e sequence of amino acids creates a different proteins.
The first primary structure of a protein i.e INSULIN was determined by Frederic Sanger (a British chemist) and for this work he was awarded the Nobel prize in 1958. The different chemical and biological properties of various proteins are primarily due to the difference in their primary structures.
Normal Haemoglobin –Val – His – Leu – Thr – Pro – Glu –Lys–
Sickle cell anemia Val – His – Leu – Thr – Pro – Val – Lys–
(ii) Secondary structure of Proteins :
The conformation in which the polypeptides chains assume a shape as a result of H-bonding is called the secondary structure of protein.
Types:
(a) a-Helix structure
(b) b-pleated sheet structure
(iii)Tertiary structure of proteins :
The tertiary structure of protein represent overall folding of the polypeptide chains i.e further folding of the secondary structure. In other words, tertiary structure refers to the manner in which the entire protein molecule folds up in the three dimensional space to produce a specific shape. It gives rise to two major molecular shapes fibrous and globular. The main forces which stabilise the 2º and 3º structures of proteins are hydrogen bonds, disulphide linkages, vander waals and electrostatic forces of attraction.
(iv) Quarternary structure of proteins :
Although many proteins exist as a single polypeptide chain but there are certain proteins which are composed of two or more polypeptide chains referred to as sub-units or protomoss. These subunits may be identical or different and are held together by non-polar covalent forces such as, H-bonds, vanderwaals interaction and electrostatic interactions. The quarternary structure refers to the determination of the number of sub-units and their special arrangement w.r.t. each other in an aggregate protein molecule The Haemoglobin possesses quarternary structure and it is an aggregate of four polypeptide chains or sub-units.
A diagramatic representation of all four structure is given below, each ball represent an amino acid.
Denaturation of proteins :
A protein found in a biological sytem having a unique three dimensional structure and specific biological activity is called a native protein. When a protein in its native form is subjected to a physical change like change in temperature or pH, the hydrogen bonds are disturbed. As a result-globules unfold and helices get uncoiled and protein loses its biological activity. This is called Denaturation of proteins. During denaturation 2º and 3º structure are destroyed but 1º structure remain intact.
- Changing the pH denatures proteins because it changes the charges on many of the side chains. This disrupts electrostatic attractions and hydrogen bonds.
- Certain reagents such as urea and guanidine hydrochloride denature proteins by forming hydrogen bonds to the protein groups that are stronger than the hydrogen bonds formed between the groups.
- Detergents such as sodium dodecyl sulphate denature proteins by associating with the non polar groups of the protein, thus, interfering with the normal hydrophobic interactions.
- Organic solvents denature proteins by disrupting hydrophobic interactions.
- Proteins can also be denatured by heat or by agitation. Both increase molecular motion, which can disrupt the attractive forces. The coagulation of egg white on boiling and curdling of milk caused by the bacteria present in milk are common examples of denaturation of protein.
Renaturation of proteins :
The denaturation may be reversible or irreversible. The coagulation of egg white on boiling and curdling of milk are an example of irreversible protein denaturation. There is some cases where the process in actually reversible. The reverse process is called renaturation. In such cases when the temperature and pH of a denatured protein are brought back, the secondary and tertiary structures of protein are restored.
Test for proteins:
(a) Biuret test : Biuret reagent is a blue colour mixture of sodium hydroxide and copper sulphate. The blue colour of the reagent turns violet in the presence of proteins.
Condition :
- At least two peptide linkages must be present.
eg. Dipeptides do not give this test but tri, tetra,..........can give this test.
(b) Xanthoprotic:
Protein + conc. HNO3 ® Orange colour alkaline solution
(c) Ninhydrin test :
Proteins or Amino acids Purple coloured product.
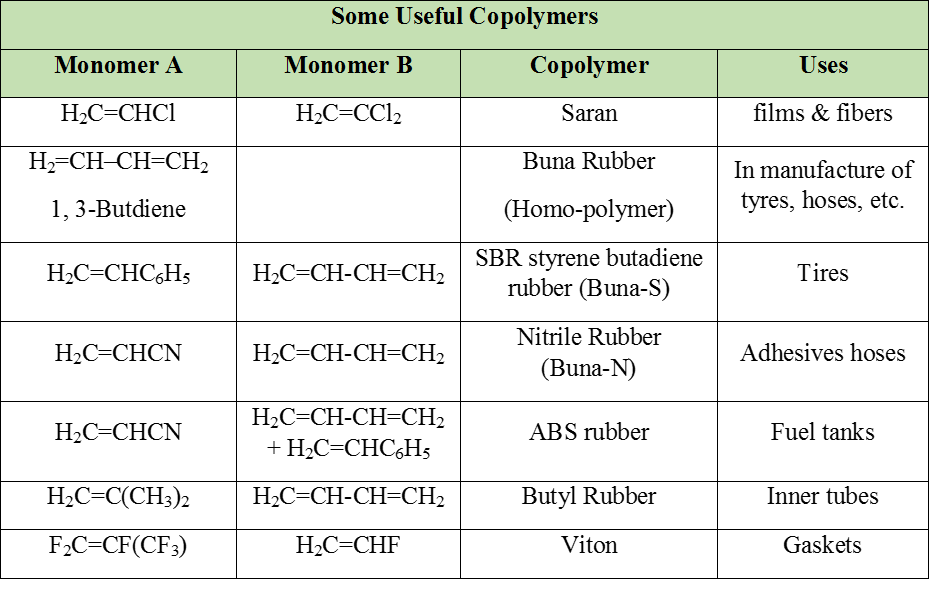
3. Polymers
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Polymers
These are very high molecular mass substances where each molecule is derived from very large number of simple molecules joined together in a regular way. This simple molecule is monomer and the process of formation of polymers from simple molecule is polymerization.
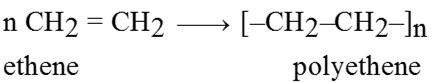
Polymers are two types :
(i) Homopolymers :
Polymers in which repeating structural units are derived from only one type of monomer units are called homopolymers.

Ex. Polypropylene, polyvinyl chloride (PVC), polyisoprene, neoprene (polychloroprene) polyacrylonitrile (PAN), nylon-6, polybutadiene, teflon (polytetrafluoroethylene), cellulose, starch etc.
(ii) Copolymers :
Polymers in which repeating structural units are derived from two or more types of monomer units are called copolymers.

Note : In Homo polymer empirical formula of monomer and polymers are same
4. Classification of Polymers
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Classification- Polymers can be classified by following ways
(A) Classification based on origin or source :
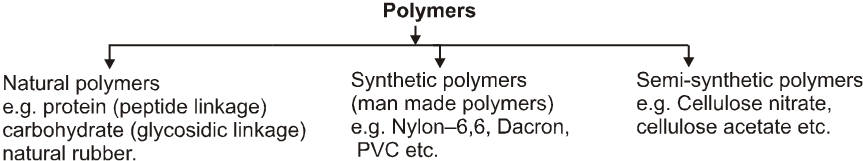
(B) Classification based on synthesis :

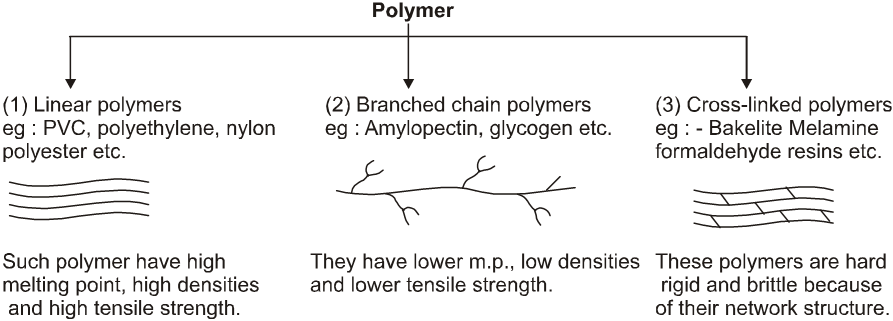
(C) Classification based on Structure:
(D) Classification based on Mechanism :
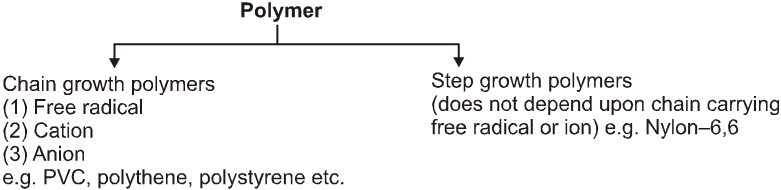
(1) Free radical :
This mechanism involve catalyst which generate free radical. Steps involved are :

(E) Calssification based upon molecular force :
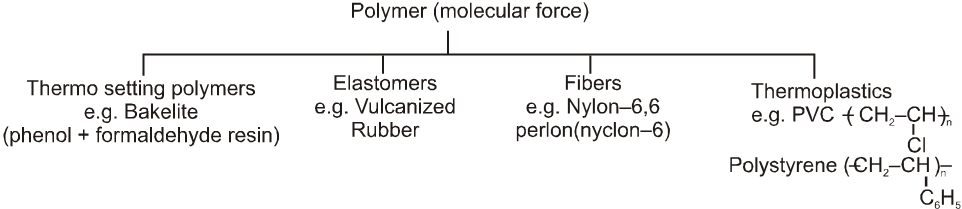
(i) Elastomers : Polymers in which the intermolecular forces of attraction between the polymer chains are the weakest are called elastomers.
e.g. Buna rubber, Natural rubber etc.
(ii) Fibres : Polymers in which the intermolecular forces of attraction are the strongest are called fibers. These forces are either due to H-bonding or dipole-dipole interactions. In case of nylon (polyamides), the intermolecular forces are due to H-bonding while in polyesters (terylene, dacron) and polyacrylonitrile (orlon, acrylin) Dipole-dipole interactions between the polar carbonyl (C = O) groups and, between carbonyl and cyano (– C º N) groups respectively.
(iii) Thermoplastics : Polymers in which the intermolecular forces of attraction are in between those of elastomers and fibres are called thermoplastics. The process of heat softening and cooling can be repeated as many times as desired without any change in chemical composition and mechanical properties of the plastic.
e.g. Polyethylene, polypropylene, polystyrene, PVC, teflon etc.
(iv) Thermosetting polymers : These are semifluid substances with low molecular weights which when heated in a mould, undergo change in chemical composition to give a hard, infusible and insoluble mass. This hardening on heating is due to extensive cross-linking between different polymer chains to give a three-dimensional network solid. Examples : Phenol-formaldehyde (bakelite), urea-formaldehyde, etc.
e.g. Bakelite urea-formaldehyde resin, terylene etc.

Natural rubber :
Natural rubber is a polymer of isoprene, and obtained from natural source-latex tree. In natural rubber, isoprene units are joined together in head-to-tail fashion and all double bonds in the polymer chain have cis configurations as shown in the given figure.
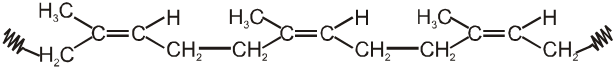
(Natural rubber)
The polymer contains cis repeating units and has a molecular weight ranging from 100,000 upto 1,000,000. A related polymer, called gutta percha which has a structure with trans double bonds and a much lower molecular weight. A typical sample of gutta percha has a molecular weight of about 7,000.
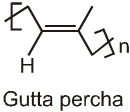
The cis arrangement of the double bonds in natural rubber prevents the rubber molecules from fitting into an ordered structure. Thus, rubber is an amorphous polymer. Because of the random coiling of its polymer chains, rubber stretches easily. When strectched, the rubber molecules are forced into a higher energy state. When the tension is released, rubber snaps back to its original random coiled state.
Vulcanization of Natural Rubber
Raw rubber is soft and tacky, they have very low mechanical strength, affected by environmental factors such as light, temperature, and oxygen and are of no use for any commercial purpose. These factors make rubber unsuitable for a number of applications. To enhance the mechanical properties and stiffness of natural rubber in 1839, charles Goodyear devised a method of reacting rubber with sulphur to form a more durable material.
Valcanization is a process in which natural rubber is heated with sulphur, where it undergo crosslinking and connect the isolated rubber chain through a network making them strong and stiff. Vulcanization forms both the cyclic structure shown on the left and the more desirable cross-linked structure shown on the right in the following figure.
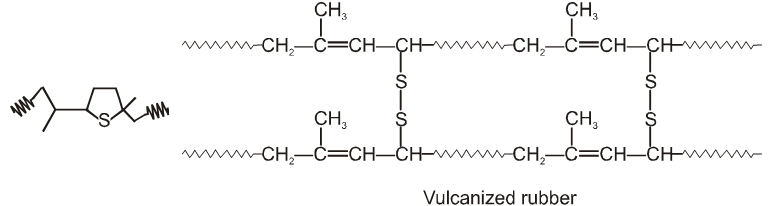
Increasing the amount of sulphur makes the vulcanized polymer harder and more durable. Adding 3-5% sulphur makes a product good for rubber bands and inner tubes. Adding 20-30% sulphur makes a hard rubber that was once widely used in ways that a hard synthetic plastic is used today. The vulcanization process made early automobile tires possible.
5. Biodegradable Polymers
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Biodegradable Polymers
A large number of polymers are quite resistant to the environmental degradation processes and are thus responsible for the accumulation of polymeric soild waste materials. These soild wastes cause acute environmental problems and remain undegraded for quite a long time. In view of the general awareness and concern for the problems created by the polymeric soild wastes, certain new biodegradable synthetic polymers have been designed and developed. These polymers contain functional groups similar to the functional groups present in biopolymers.
Aliphatic polyesters are one of the important classes of biodegradable poylmers. Some examples are given below :
(A) Poly b-hydroxybutyrate – co–b-hydroxy valerate (PHBV) :
It is obtained by the copolymerisatin of 3-hydroxybutanoic acid. PHBV is used in speciality packaging, orthopaedic devices and in controlled release of drugs. PHBV undergoes bacterial degradation in the environment.
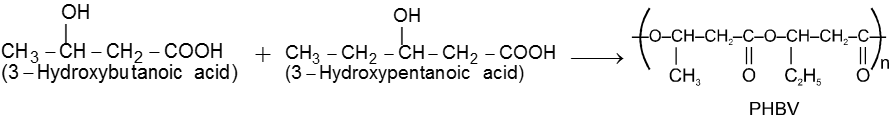
(B) Nylon–2–nylon–6 :
It is an alternating polyamide copolymer of glycine (H2N–CH2–COOH) and amino caproic acid
[H2N (CH2)5 COOH].
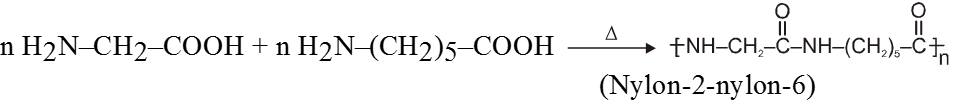
It is step-growth copolymer.
(C) Poly glycolic acid (PGA) and poly lactic acid (PLA) :- This copolymer is commercially called dextron.
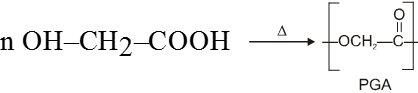
Glycolic acid
A copolymer of PGA and PLA (90 : 10) was the first biodegradable polyester used for stitching of wounds after operation.
(D) Poly-S-caprolactone lactone (PCL) : - It is obtained by chain polymerization of the lactone of 5(or S) hydroxy hexanoic acid.
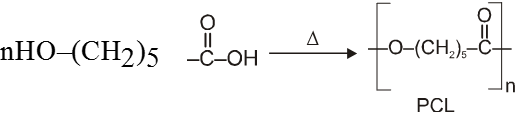
5(or S-) hydroxy hexanoic acid

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
