1. Strucutre and bonding in aldehydes and ketones
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Chapter 12: Aldehydes, Ketones and Carboxylic Acids
Aldehydes and Ketones
Introduction
Aldehydes & ketones have general formula CnH2nO and contains >C = O group. Thus aldehydes (R–CHO) and ketones (R–CO–R) are collectively called as carbonyl compounds. Aldehyde is always at terminal position while ketone is never at terminal position.
Structure and bonding in aldehydes and ketones
The carbonyl carbon atom is sp2 hybridized. The un hybridized p-orbital overlaps with a p-orbital of oxygen to form a pi bond. The double bond between carbon and oxygen is shorter, stronger, and polarized.
Orbital diagram for the formation of carbonyl group is as follows:
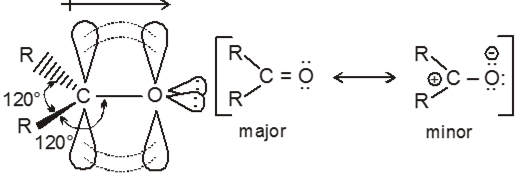
This polarity confirms that there is nucleophilic addition reaction takes place in carbonyl compound.
The double bond of the carbonyl group has a large dipole moment because oxygen is more electronegative than carbon.
Carbonyl carbon act as an electrophile (Lewis acid)
Carbonyl oxygen act as a nucleophile (Lewis base)
2. Preparation methods of Aldehydes and Ketones
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Preparation methods of Aldehydes and Ketones
By oxidation of alcohols :
Primary alcohols ![]() Aldehydes
Aldehydes
Secondary alcohols ![]() Ketones
Ketones
By dehydrogenation of alcohols :
Dehydrogenation means removal of hydrogen and reagent used is heated copper.
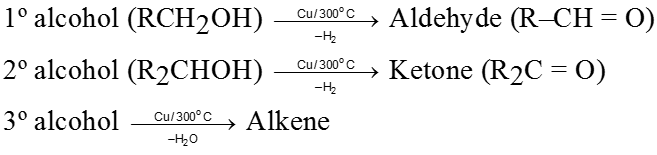
Ozonolysis of alkene :
It is used to get carbonyl compounds from alkene. The reaction is
Note :
(i) During the cleavage of ozonide Zn is used to check further oxidation of aldehyde into acid.
(ii) By this method we can locate double bond in olefin or exact structrue of hydrocarbon can be determined by knowing ozonolysis product i.e. by placing double bond at the place of two carbonyl oxygen atoms of two carbonyl compounds.
(iii) Among the three molecules of carbonyl compounds.
(a) If one molecule contains two carbonyl groups, then hydrocarbon will be alkadiene.
(b) If all the three molecules contain two carbonyl group then hydrocarbon will be cycloalkatriene.
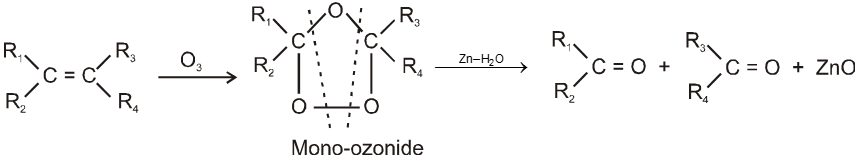
Wacker process :
Alkenes can directly be oxidised to corresponding aldehydes or ketones by treating them with a solution of PdCl2 containing a catalytic amount of CuCl2 in presence of air or O2 . Except ethene any higher alkene will give ketone.
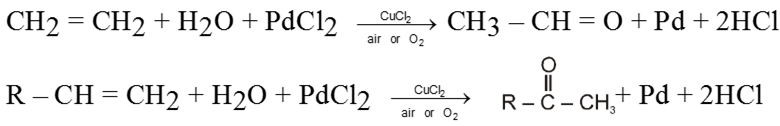
Note : During the reaction PdCl2 is reduced to Pd and CuCl2 is reduced to Cu(I)
Hydration of alkynes :
![]()
Other alkynes give ketones in this reaction.
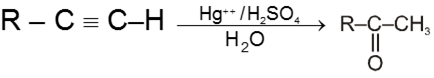
Hydroboration of alkyne :
It is used to get aldehyde from terminal alkyne. Here reagent is (i) diborane (B2H6) (ii) H2O2,OH–
![]()
In this reaction Borane (BH3) is act as electrophile.
Dry distillation of calcium salt of acid :
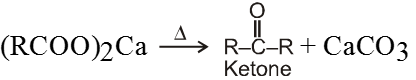
Ex.
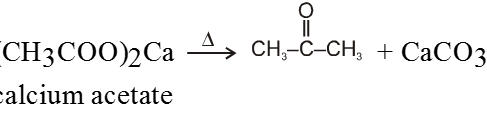
On dry distillation of calcium salt of acetic acid with calcium salt of formic acid we get a mixture of aldehyde, ketone and formaldehyde.
On passing vapours of fatty acids over Mangnous oxide at 300ºC :
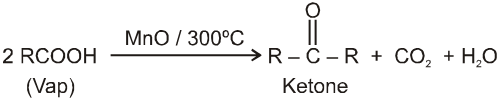
On passing mixture of vapours of fatty acid with formic acid we get a mixture of aldehyde, ketone and formaldehyde.
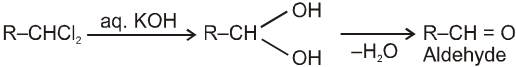
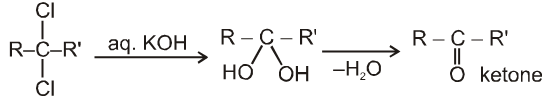
On aqueous alkali hydrolysis of gem-dihalides :
Terminal gemdihalides will give aldehyde while non-terminal will give ketone as follows
3. Methods used for the preparation of Aldehydes only
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Methods used for the preparation of Aldehydes only
Rosenmund's reduction :
Here acid chlorides are reduced to aldehyde with H2 in boiling xylene using palladium as a catalyst supported on barium sulphate.
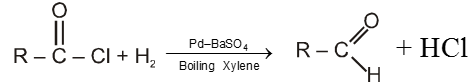
Note :
(a) Pd Catalyst is poisoned by BaSO4 to check further reduction of aldehyde to alcohol.
(b) Formaldehyde cannot be obtained by this method because HCOCl is unstable at common temperature.
(c) Reaction with acid chloride and dialkyl cadmium we can obtain ketone.
Stephen's reduction :
![]()
Oxo-process :
It is also called as carbonylation here alkene reacts with water gas at high temperature and pressure in the presence of cobalt carbonyl catalyst to give aldehyde.
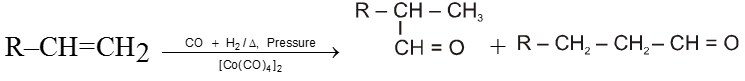
Reimer-Teimann Reaction :
By this method phenolic aldehyde is prepared

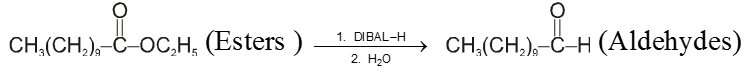
From esters or nitrile :
![]()
DIBAL–H : Diisobutyl aluminium hydride [AlH(i-Bu)2] is a reducing agent.
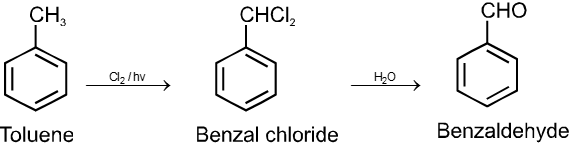
From hydrocarbons :
By oxidation of methyl benzene and its derivative using chromyl chloride (CrO2Cl2)
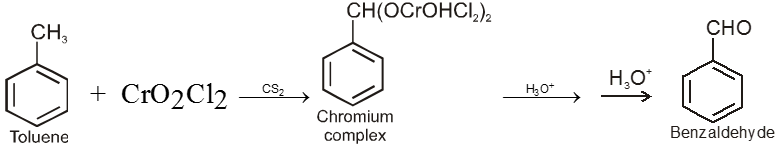
This reaction is called Etard reaction.
By oxidation of methyl benzene and its derivative using chromic oxide (CrO3) in acetic anhydride:

By Gatterman-Koch reaction :
![]()
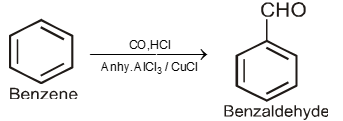
4. Methods used for the preparation of Ketones only
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Methods used for the preparation of Ketones only
Using alkanoylchloride and dialkyl cadmium :
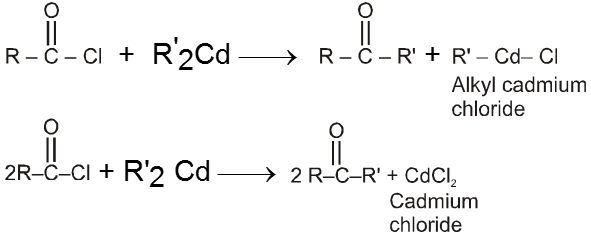
By acylation or benzoylation of aromatic hydrocarbon (Friedel-Craft Reaction)
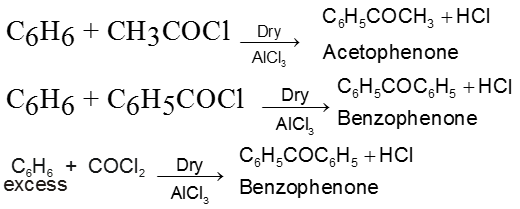
By acid hydrolysis followed by heating of b-Ketoester :
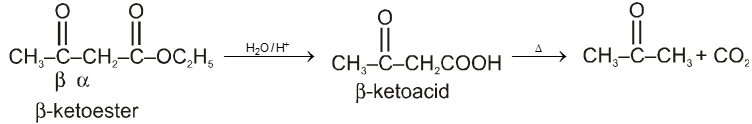
Note : It is b-ketoacid which decarboxylate more readily as it proceeds via six membered cyclic transition-state.
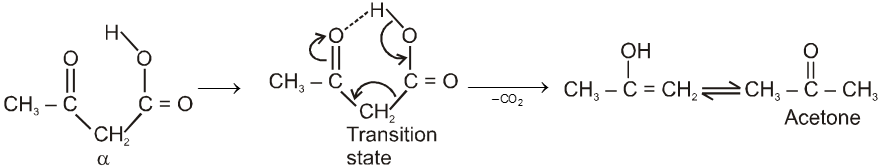
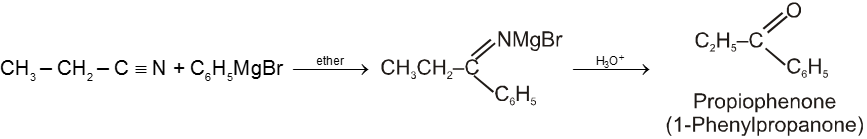
From nitriles :
Treatment of nitrile with Grignard reagent followed by hydrolysis gives a ketone.

5. Physical properties of Aldehydes and Ketones
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Physical properties of Aldehydes and Ketones
Physical properties of Aldehydes and Ketones :
- Methanal - Gas at room temperature
- Ethanal - Volatile liquid
- Other aldehydes and ketones - Liquid or solid at room temperature
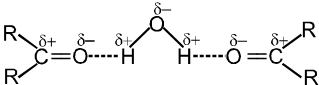
- Boling points of aldehydes and ketones are higher than those of hydrocarbons and ethers of comparable molecular masses.
Reason : Weak molecular association in aldehydes and ketones, arising out of the dipole-dipole interactions
- Boiling points of aldehydes and ketones are lower than those of alcohols of similar molecular masses.
Reason : Absence of intermolecular hydrogen bonding
- Lower members of aldehydes and ketones are miscible with water in all proportions.
Reason : They form hydrogen bonds with water.
- Solubility of aldehydes and ketones decreases rapidly on increasing the length of the alkyl chain.
- All aldehydes and ketones are fairly soluble in organic solvents such as ether, methanol, etc.
- Lower aldehydes have sharp pungent odours.
- As the size of aldehydes increases, the odour becomes less pungent and more fragrant.
6. Chemical Reactions
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Chemical Reactions
Nucleophilic addition reactions :
Addition of a nuceophile and a proton across the (C = O) double bond. The reactivity of the carbonyl group arises from the electronegativity of the oxygen atom and the resulting polarization of the carbon-oxygen double bond. The electrophilic carbonyl carbon atom is sp2 hybridized and flat, leaving it relatively unhindered and open to attack from either face of the double bond.
Mechanism :
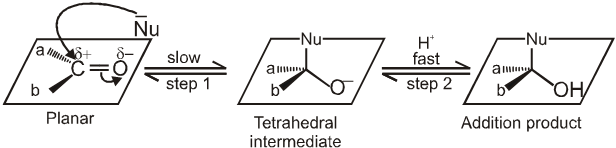
Nucleophile (Nu-) attacks the carbonyl group perpendicular to the plane of sp2 hybridised orbitals of carbonyl carbon.
In the process, hybridisation of carbon changes from sp2 to sp3.
A tetrahedral alkoxide is formed as intermediate.
Reactivity : Aldehydes are more reactive than ketones in nucleophilic addition reactions.
![]()
There are two factors which influence the reactivity of ketone and aldehyde.
(i) Inductive effect (ii) steric factor
(i) + I effect of alkyl group decrease the amount of charge on C+ (C+ – O–). in ketones.
ii) Steric effect also causes the less reactivity of carbonyl group.
(I) Addition of hydrogen cyanide (HCN)
![]()
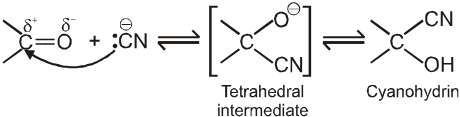
Note :
(i) Addition of HCN over aldehyde and ketones gives cyanohydrin.
(ii) Cyanohydrin on acid hydrolysis gives a-hydroxy acid.
(iii) Cyanohydrin on treating with NH3(l) followed by acid hydrolysis gives a-amino acid.
(iv) In case of ketone cyanohydrin formation is reversible due to bulky group of ketone which hinder the formation.
(II) Addition of sodium hydrogen sulphite (NaHSO3)
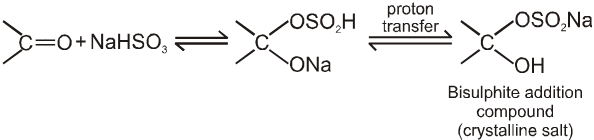
salt on hydrolysis gives carbonyl compounds again, this reaction is used to separate the aldehydes from mixture.
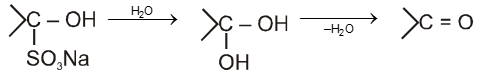
(III) Addition of alcohols (ROH) :
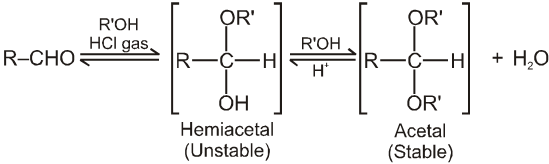
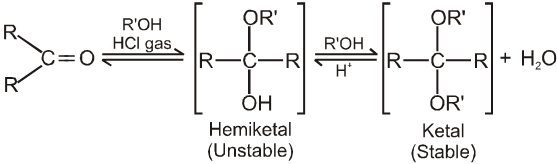
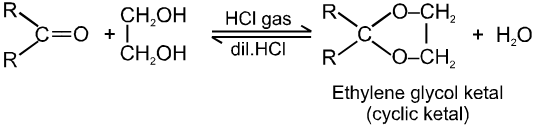
Note :
(i) Acetal is formed to protect aldehyde for a long time.
(ii) Acetal has functional groups ether.
(iii) Acetal formed can be decomposed to original aldehyde by dilute acid.
(iv) On treating with ethyleneglycol we get cyclic acetal or ketal.
(v) Acetal formation is found to be more favourable than ketal formation if both the carbonyl groups are present within the molecule.
(IV) Addition of water :
Aldehyde or ketone reacts with water to form gem-diols. Water is a poor nucelophile and therefore adds relatively slowly to the carbonyl group, but the rate of reaction can be increased by an acid catalyst.
Mechanism:
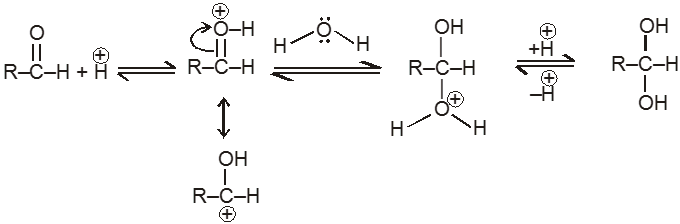
(V) Addition of ammonia and its derivatives (addition elimination reactions) :
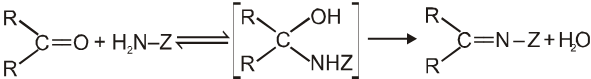
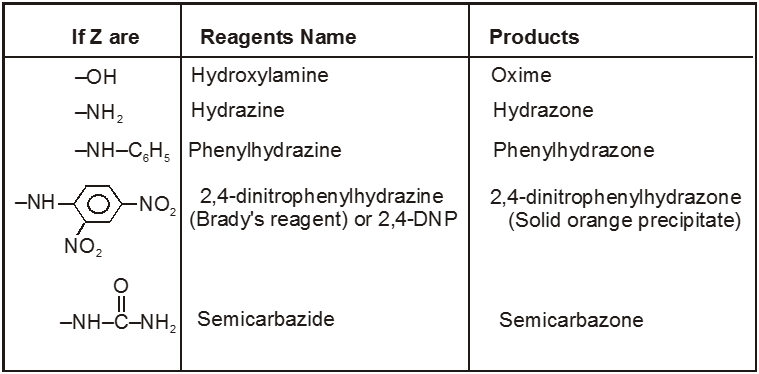
(VI) Addition of Grignard reagents (Preparation of alcohol) :
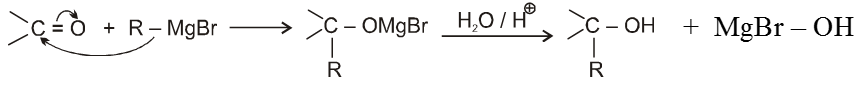
(a) When formaldehyde is treated with Grignard reagent followed by acid hydrolysis primary alcohol is obtained.
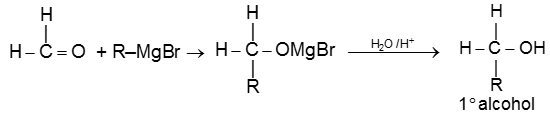
(b) When aldehyde except formaldehyde is treated with grignard reagent followed by hydrolysis 2° alcohol is obtained.
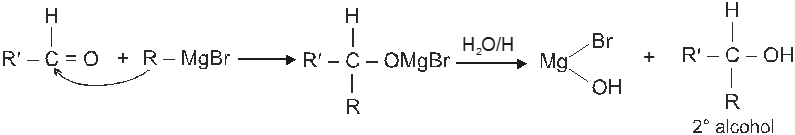
(c) When ketone is treated with grignard reagent followed by acid hydrolysis 3° alcohol is obtained.
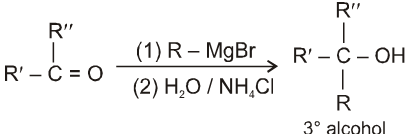
Beckmann rearrangement in Oximes:
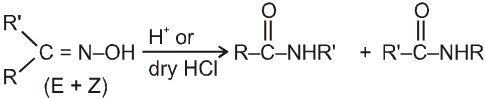 (If R' is bulkier than R)
(If R' is bulkier than R)
Mechanism :
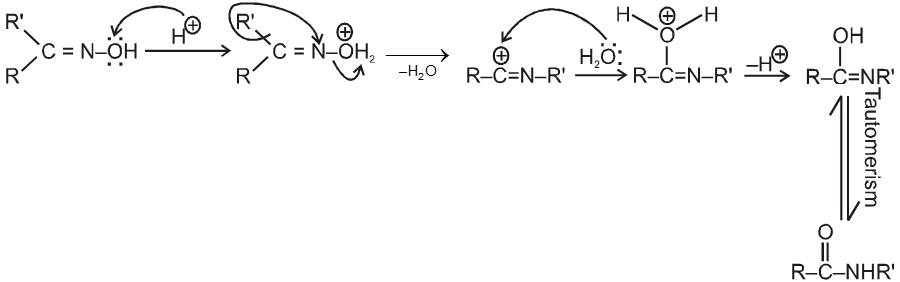
Note :
(i) Oxime undergoes Beckmann rearrangement to give its isomer amide.
(ii) In this reaction the group which is anti to –OH group migrates.
Ex.
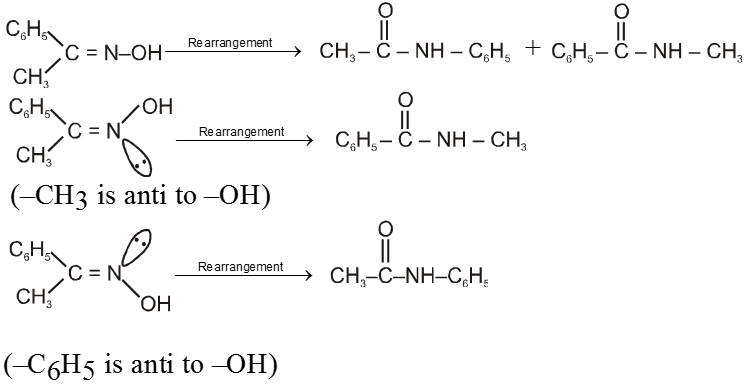
Reactions due to a-Hydrogen
a-Hydrogen of aldehydes and ketones are acidic: They undergo a number of reactions due to the acidic nature of a-hydrogen.
Reason for the acidity of a-hydrogen: Strong electron-withdrawing effect of the carbonyl group, and resonance stabilisation of the conjugate base
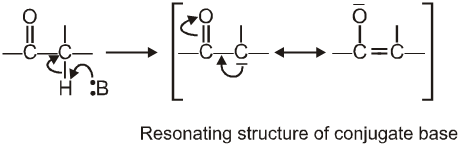
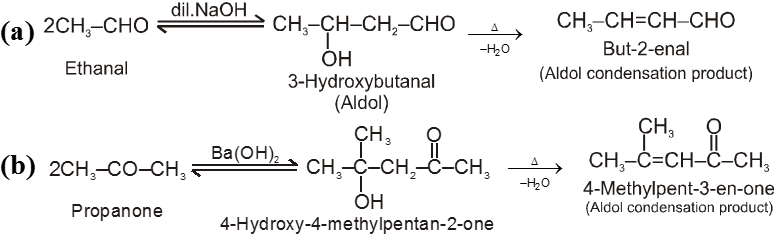
(I) Aldol condensation (or aldol reaction)
Aldehydes and ketones with at least one a-hydrogen undergo a reaction in the presence of dilute alkali as catalyst.
Mechanism
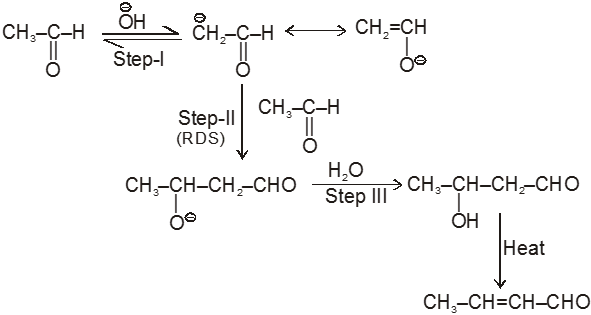
Ex.
(II) Cross-Aldol condensation :
On using two types of carbonyl compounds both having a-hydrogen atoms we get a mixture of four condensed product because two types of carbonyl compounds will give two type of carbanions which will be nucleophile for itself and other molecule.
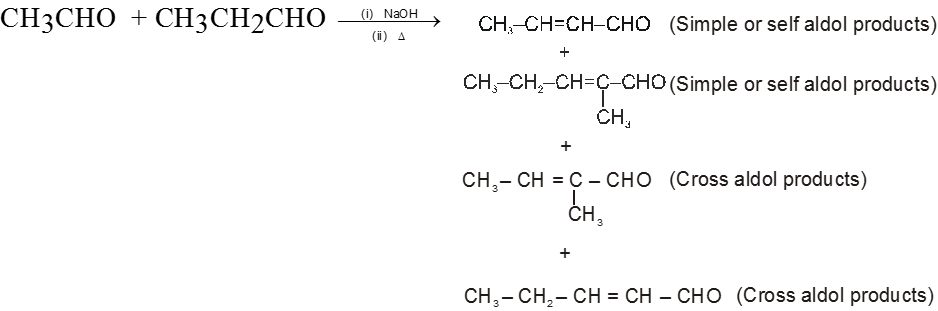
Ketones can also be used as one component in cross-aldol reactions.
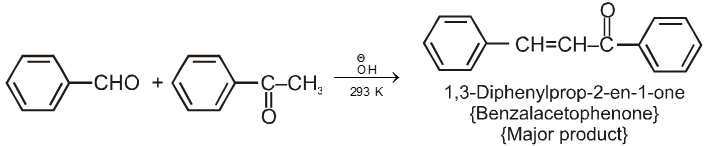
Note : On using formaldehyde and acetaldehyde during crossed aldol all the a-hydrogen atom of acetaldehyde are replaced one by one by hydroxymethyl group because of smaller size of formaldehyde to give trihydroxymethylacetaldehyde which undergoes crossed cannizaro's reaction with formaldehyde to give tetrahydroxymethyl methane and formate ion as a final product.
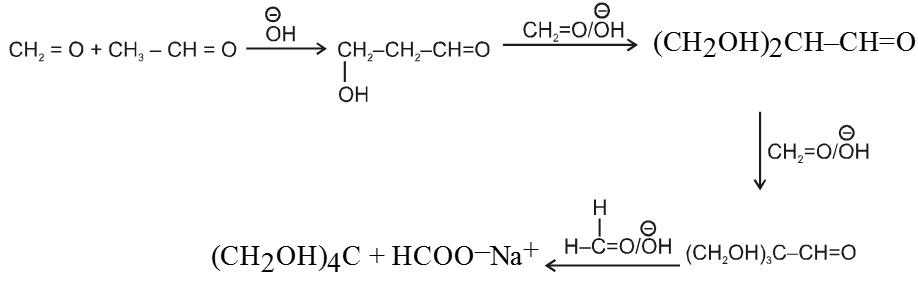
Ex. Show how cinnamaldehyde is prepared by crossed aldol condensation ?
Sol. ![]()
(III) Intramolecular aldol condensation :
If two carbonyl groups with a-hydrogen atoms are present within the same molecule, then we get cyclic a, b-unsaturated aldehyde / ketones via the formation of cyclic-b-hydroxy aldehyde / ketone in presence of basic medium.
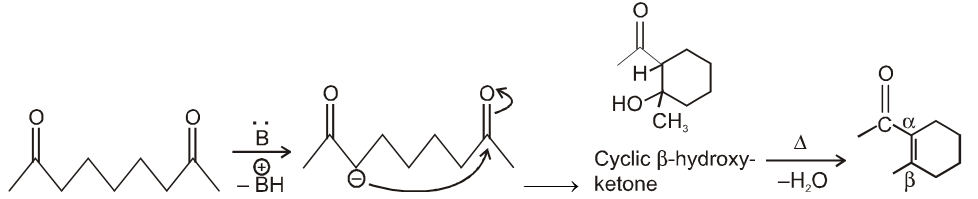
By knowing product we can get reactant as in case of intermolecular aldol condensation :
Perkin reaction :
When aromatic aldehyde like benzaldehyde is treated with anhydride in the presence of sodium salt of acid from which anhydride is derived we get a, b-unsaturated acid.

Mechanism :
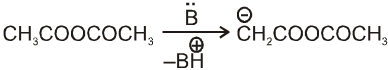
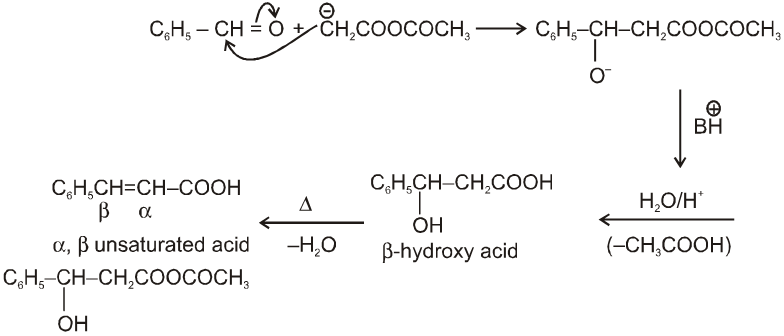
Note : By knowing a, b-unsaturated acid we can get idea about the anhydride used in perkin reaction. This can be done by keeping 'H' at a and –OH at b-carbon atom followed by breaking a, b- carbon. By this we can know about acid and it will be anhydride of this acid only.
Cannizzaro reaction :
Aldehydes which do not have an a- hydrogen atom, undergo self oxidation and reduction (disproportionation) reaction on treatment with a concentrated alkali.
Ex.
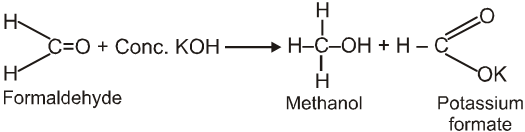
Mechanism :
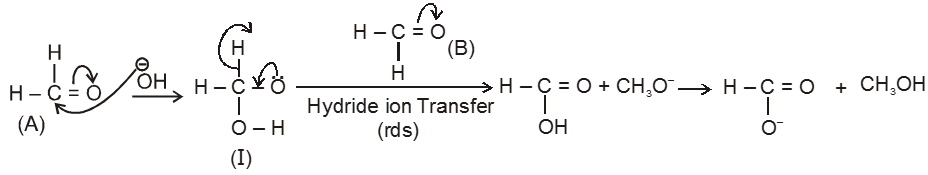
By this mechanism it is clear that acid corresponds to that carbonyl compound over which ![]() can attack easily as nucleophile.
can attack easily as nucleophile.
Note : It is observed that hydride ion transfer from (I) to Carbonyl compound (B) is rate determining step.
Crossed Cannizzaro reaction :
On using two types of carbonyl compounds not having a-hydrogen atom, acid salt will be corresponding to that aldehyde over which ![]() will approach without any hindrence.
will approach without any hindrence.
(i) 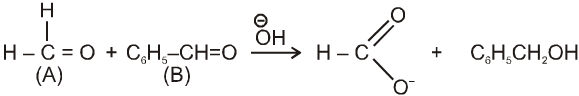
(ii) 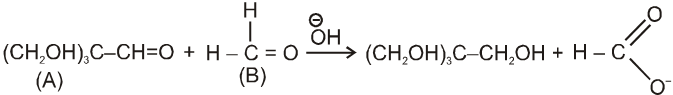
in case (i) ![]() will easily go to (A) and in case (ii) it will go to (B) hence acid salt will be formate ion in both the cases.
will easily go to (A) and in case (ii) it will go to (B) hence acid salt will be formate ion in both the cases.
Intramolecular Cannizzaro reacion :
Here two carbonyl groups (without a-hydrogen atom) are present within the same molecule.
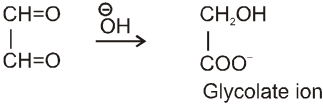
Mechanism :
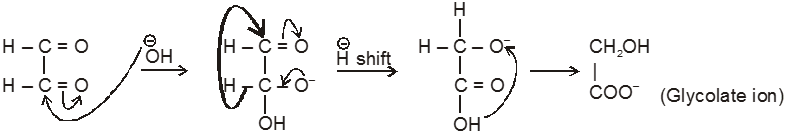
Ex.
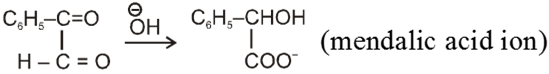
Wittig reaction :
It is used to get alkene from carbonyl compound using phosphorus ylide via the formation of cyclic structure betaine.
Mechanism :
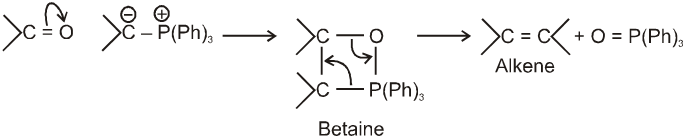
Note : Phosphorus ylides are prepared from alkylhalide and triphenylphosphine in the presence of base like sodium ethoxide as –
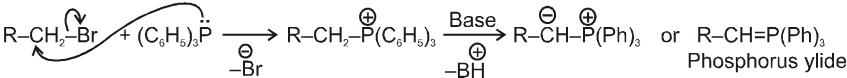
Ex.
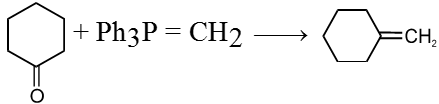
Reduction reactions
(I) Reduction to alcohols :
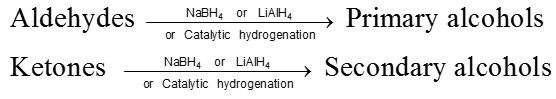
(II) Clemmensen reduction :
Used to get alkane from carbonyl compounds.
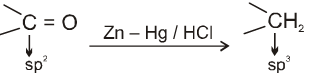
Note : Generally this reaction is avoid if acid sensitive groups are present in the carbonyl compounds.
(III) Wolf-Kishner reduction :
Used to get alkane from carbonyl compounds.
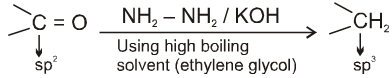
Note : Generally this reaction is avoid if base sensitive groups are present in the carbonyl compounds
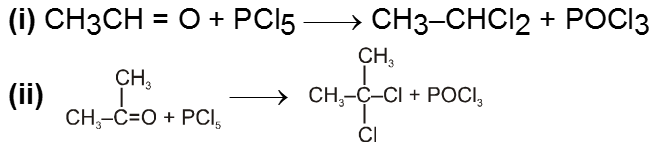
(IV) Reaction with PCl5 :
Carbonyl compounds give gemdihalides
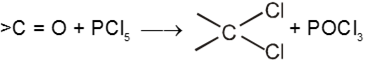
(V) Pinacol-Pinacolone rearrangement :
Pinacole is obtained when 2 moles of acetone are heated with divalent active metal magnesium followed by treating with water.
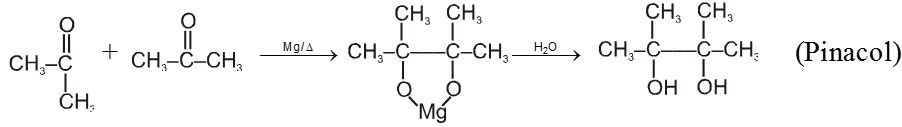
Pinacole undergoes rearrangement in acidic media to give pinacolone
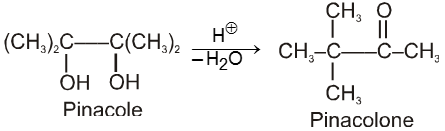
Oxidation reactions
(I) Haloform reaction :
Acetaldehyde and methylalkyl ketones react rapidly with halogen (Cl2, Br2 or I2) in the presence of alkali to give haloform and acid salt.
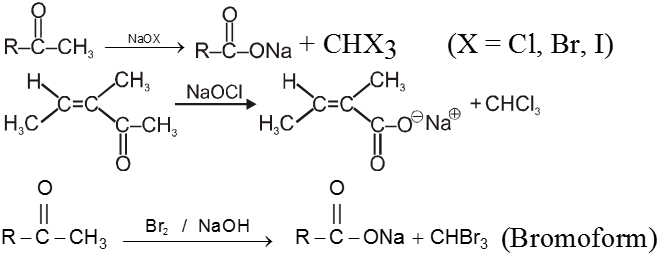
In this reaction – CH3 of 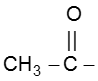 group is converted into haloform as it contains acidic hydrogen atom and rest-part of alkyl methyl ketone give acid salt having carbon atom corresponding to alkyl ketone.
group is converted into haloform as it contains acidic hydrogen atom and rest-part of alkyl methyl ketone give acid salt having carbon atom corresponding to alkyl ketone.
Preparation of haloform from methylketone involves two steps.
(a) Halogenation
(b) Alkalihydrolysis
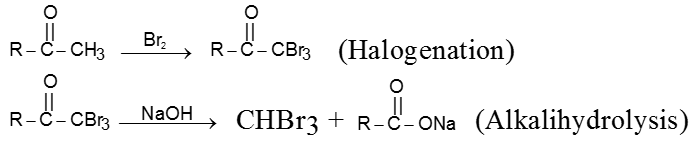
Note : This reaction is used to distinguish the presence of 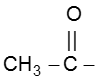 group.
group.
(II) Oxidation of Aldehydes :
• Aldehydes are oxidised to carboxylic acids by common oxidising agents such as KMnO4, HNO3, K2Cr2O7, etc.
![]()
• Aldehydes are also oxidised by mild oxidising agents such as Tollen’s reagent and Fehling’s reagent. On the other hand, ketones are not oxidised by mild oxidising agents.
• Ketones are oxidised under vigorous conditions, i.e., by strong oxidising agents and at elevated temperatures.
It involves carbon-carbon bond cleavage.
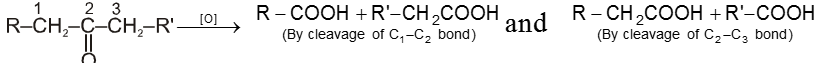
(a) Tollen’s reagent :
It is ammonical silver nitrate solution, prepared by adding ammonium hydroxide to AgNO3 solution. During reaction, first Ag2O is formed which is dissolved in ammoniumhydroxide to give Tollen’s reagent.
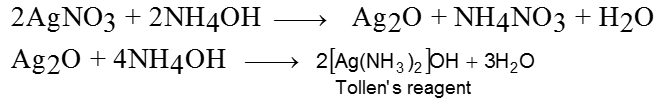
Tollen’s reagent is weak oxidising agent. It gives Ag mirror test with aldehyde.

(b) Fehling’s solution :
It is an alkaline solution of cupric ion complexed with sodium potassium tartarate.
There are two solutions in Fehling solution
Solution (A) CuSO4 solution and
Solution (B) Alkaline solution of sodium potassiumtartarate.
When these two solutions are mixed we get deep blue coloured solution.
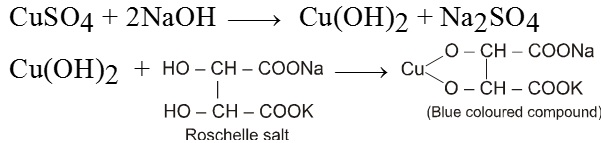
Equal volume of both the solutions are heated with aldehyde to give red brown precipitate of cuprous oxide (Cu2O) which confirms the presence of aldehyde
![]()
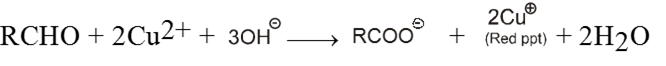
(c) Benedict solution :
It also consists of two solutions.
Solution (A) CuSO4 solution and
Solution (B) Alkaline solution of sodium Citrate.
CuSO4 + 2NaOH Cu(OH)2 + Na2SO4
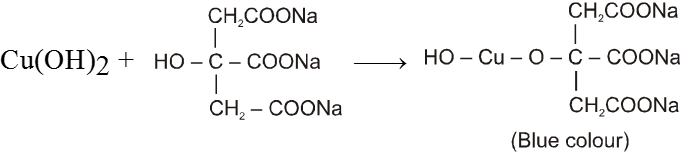
Aldehyde gives positive test with Benedict solution.
![]()
(d) Schiff’s reagent :
It is dilute solution of rosaniline hydrochloride whose pink colour has been discharged by passing SO2. Aldehyde restores pink colour when treated with schiff’s reagent (Magenta solution in H2SO3).
Other miscellaneous reactions :
(I) 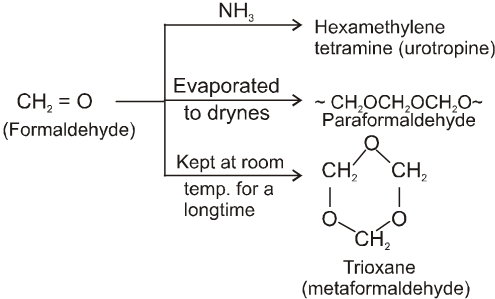
(II) 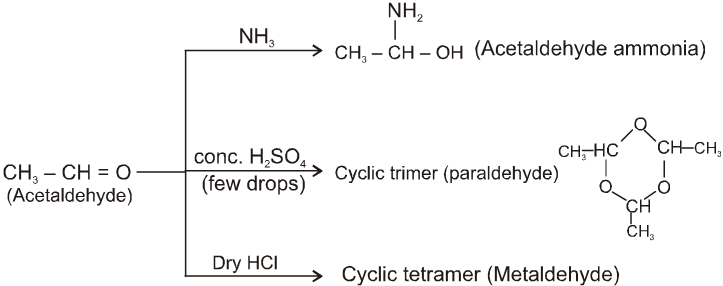
(III) 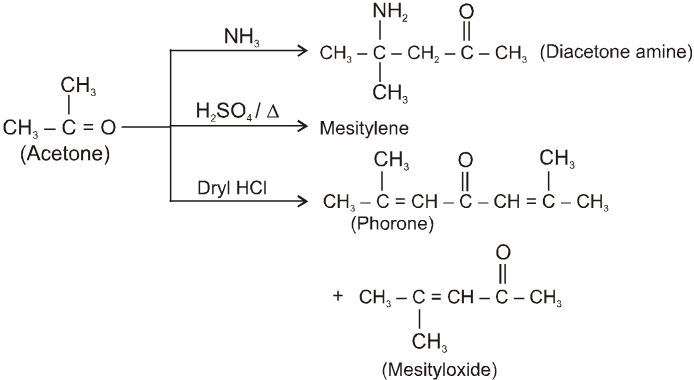
(IV) 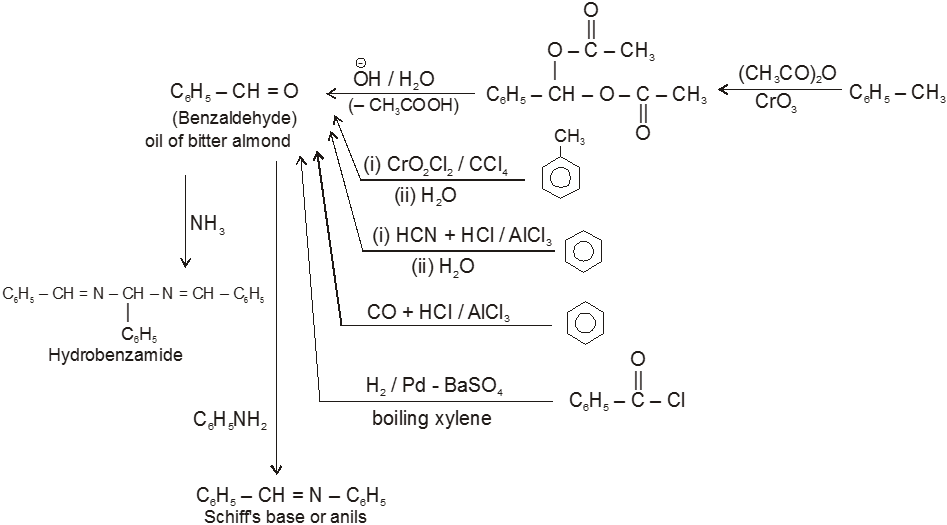
(V) 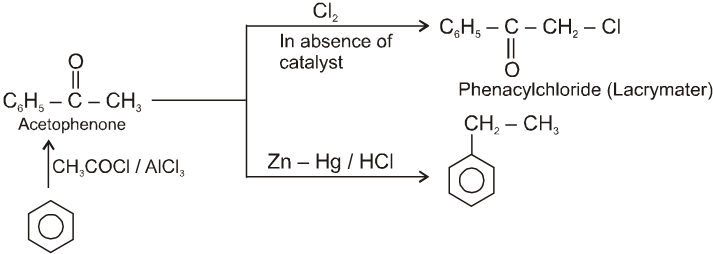
7. Uses of Aldehydes and Ketones
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Uses of Aldehydes and Ketones
- Act as solvents.
- Act as starting materials and reagents for the synthesis of other products.
- Formalin (40% solution of formaldehyde)- Used for preserving biological specimens manufacturing of bakelite, urea , formaldehyde glues and other polymers products.
- Acetaldehyde used in the manufacture of acetic acid, ethyl acetate, vinyl acetate, polymers and drugs.
- Benzaldehyde used in perfumery and in dye industries.
- Butyraldehyde, vanillin, camphor, etc., are well known for their odours and flavours.
- Acetone and ethyl methyl ketone are common industrial solvents.
8. Carboxylic acid and derivatives of carboxylic acids
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Carboxylic acid and derivatives
Introduction :
Compounds containing the carboxyl group are distinctly acidic and are called carboxylic acids.There general formula is CnH2nO2.
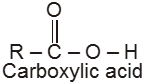
Carboxylic acid derivatives are compounds with functional groups that can be converted to carboxylic acids by a simple acidic or basic hydrolysis. The most important acid derivatives are esters, amides, nitriles, acid halides and anhydrides.
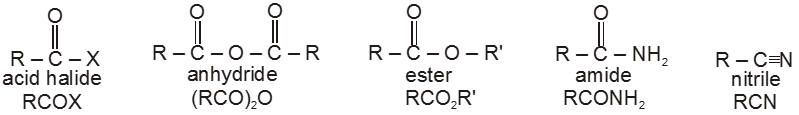
Dicarboxylic acids :
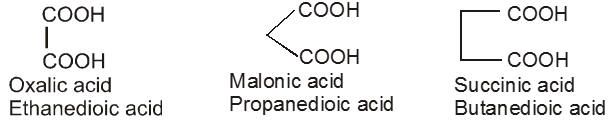
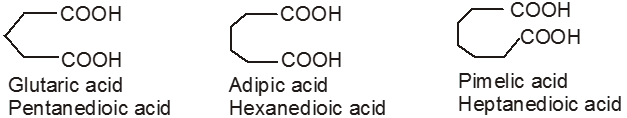
If the compound containing two carboxyl groups, these are known as dicarboxylic acid.
For example :
Physical properties of acids and acid derivatives :
(1) Boiling point : The boiling point of carboxylic acids are higher than that of alcohols, ketones or aldehydes of similar molecular weight.
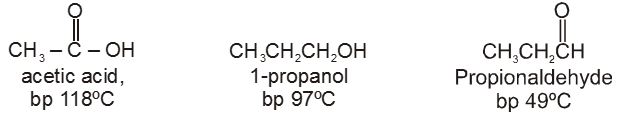
The high boiling points of carboxylic acids is the result of formation of a stable hydrogen-bonded dimer.
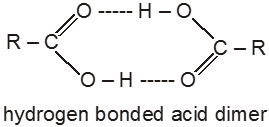
(2) Melting points :
Melting point of carboxylic acids : There is no regular pattern in melting point of carboxylic acid (up to 10 carbon atoms) having even number of C atoms are higher than neighbouring members having odd number of C atoms because carboxylic acid and methyl group in even members lie in opposite side of zig-zag carbon chain hence they fit better into crystal lattice resulting in higher melting points.Vice-versa is observed in case of carboxylic acid having odd no. of carbon atoms.

Amides have surprisingly high boiling points and melting points compared with other compounds of similar molecular weight. Primary and secondary amides participate in strong hydrogen bonding.
(3) Solubility:
Carboxylic acids form hydrogen bonds with water and the lower molecular - weight carboxylic acids (upto 4 carbon atoms) are miscible with water.
Acid derivatives (esters, acid chlorides, anhydrides, nitriles and amides) are soluble in common organic solvents such as alcohols, ethers, chlorinated alkanes and aromatic hydrocarbons. Acid chlorides and anhydrides cannot be used in nucleophilic solvents such as H2O and alcohols, because they react with these solvents.
(4) Aliphatic carboxylic acids upto nine carbon atoms are colourless liquids at room temperature, higher acids are wax like solids due to their low volatility.
9. Methods of Preparation of Carboxylic Acid
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Methods of Preparation of Carboxylic Acid
1. From primary alcohols
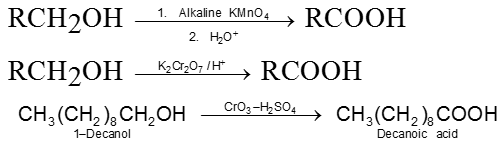
2. From aldehydes
![]()
Oxidising agents - HNO3, KMnO4, K2Cr2O7
Mild oxidising agents - Tollen’s reagent and Fehling’s reagent
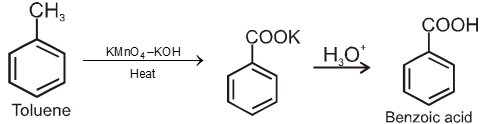
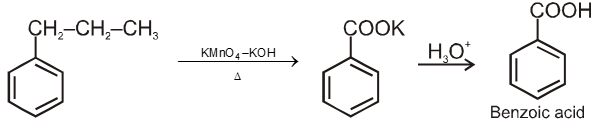
3. From alkyl benzenes
![]()
1° and 2° alkyl benzene are oxidised in this manner. Tertiary group is not affected.
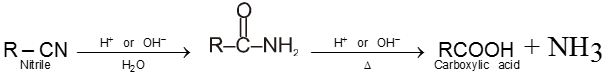
4. From nitriles and amides
5. From Grignard reagents
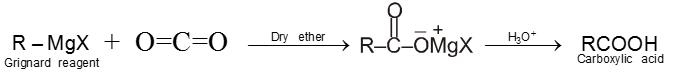
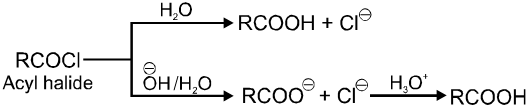
6. From acyl halides
7. From acyl anhydrides
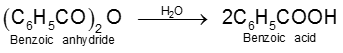
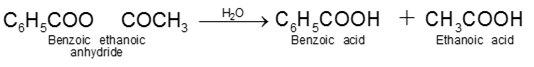
8. From esters
![]()
Ex.
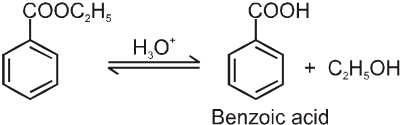
![]()
Ex. 
10. Chemical Reactions of carboxylic acid
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Chemical Reactions of carboxylic acid
Acidic Nature : Acidity of carboxylic acids :
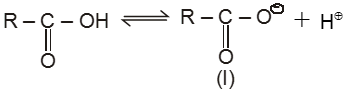
(i)![]()
(I) exists as two equivalent canonical structures I(A) and I(B). This ion is resonance stablised and resonance hybrid structure is I(C).
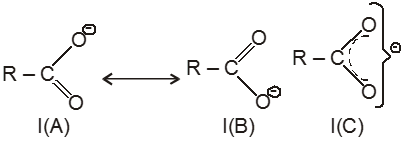
(ii) ion is more stable due to resonance, hence carboxylic acids are acidic in nature.
(iii) Electron withdrawing group (–I effect) stablises the anion and hence, increases acidic nature.
Ex. 
Ex. 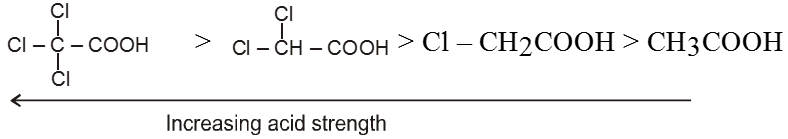
(iv) Electron releasing group (+ I effect) destablises the anion and hence decreases acidic nature.
Ex. HCOOH > CH3COOH > CH3 – CH2 – COOH
Ex. 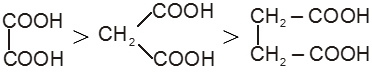
Direct attachement of groups such as phenyl or vinyl to the carboxylic acid increases its acidity.
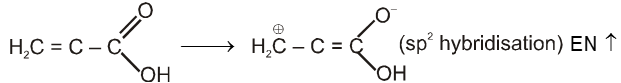
Ex. Relative acid strength is: RCOOH > HOH > ROH > HCCH > NH3 > RH
Note : Acidity of acids is compared by compairing stability of conjugate base.
(2) Reaction involving removal of proton from –OH group :
(i) Action with blue litmus : All carboxylic acids turn blue litmus into red.
(ii) Reaction with metals :
![]()
![]()
(iii) Reaction with alkalies :
CH3COOH + NaOH¾® CH3COONa + H2O
(iv) Reaction with carbonates and bicarbonates :
2CH3COOH + Na2CO3¾®2CH3COONa + CO2 + H2O
CH3COOH + NaHCO3¾®CH3COONa + CO2 + H2O
Reaction of carboxylic acid with aqueous sodium bicarbonate solution produces brisk effervescence. However most phenols do not produce effervescence. Therefore, the reaction may be used to distinguish between carboxylic acids and phenols.
(v) Reaction with grignard reagent :
![]()
Feasible reaction : A stronger acid displaces a weaker acid from the salt of the weaker acid.
Ex. CH3COOH (Stronger acid) + CH3ONa¾® CH3COONa + CH3–OH (Weaker Acid)
Ex. CH3COOH (stronger acid) + NaHCO3¾® CH3COONa + H2CO3 (Weaker acid) ¾® H2O + CO2![]()
(lab. test of carboxylic acid)
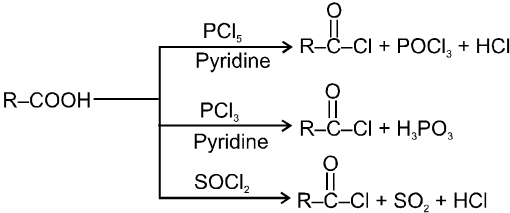
(3) Reaction involving replacement of –OH group :
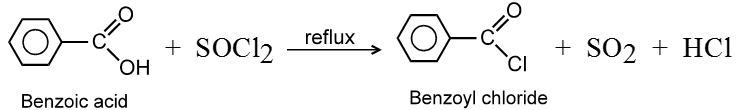
(i) Formation of acid chlorides :
Ex.
Ex.
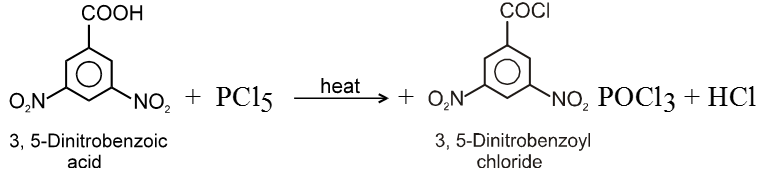
(ii) Fisher Esterification :
Carboxylic acid react with alcohol to form esters through a condensation reaction known as esterification.
General Reaction :
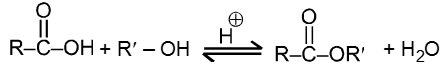
Specific Examples:
![]()
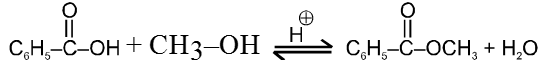
Mechanism : (Acid catalysed esterification)
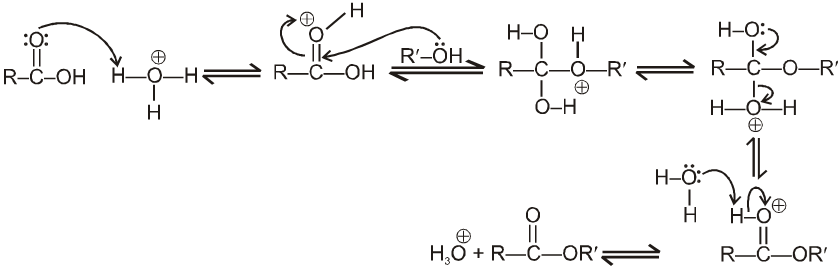
If we follow the forward reactions in this mechanism, we have the mechanism for the acid catalysed esterification of an acid. If however, we follow the reverse reactions, we have the mechanism for the acid catalysed hydrolysis of an ester.
Acid catalysed ester hydrolysis.
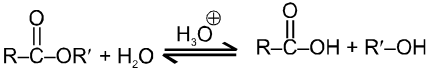
Which depends upon condition we choose. If we want to esterify an acid, use an excess amount of the alcohol and, if possible remove the water as it is formed.
If we want to hydrolyse an ester, we use a large amount of water i.e. dil HCl or dil H2SO4.
(iii) Formation of amides :
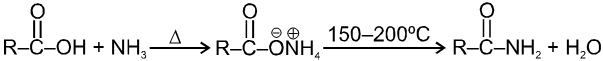
In fact amides can not be prepared from carboxylic acids and amines unless the ammonium salt is heated strongly to dehydrate it. This is not usually a good method of preparing amides.
(iv) Formation of acid anhydride :
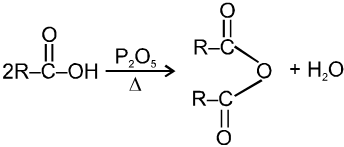
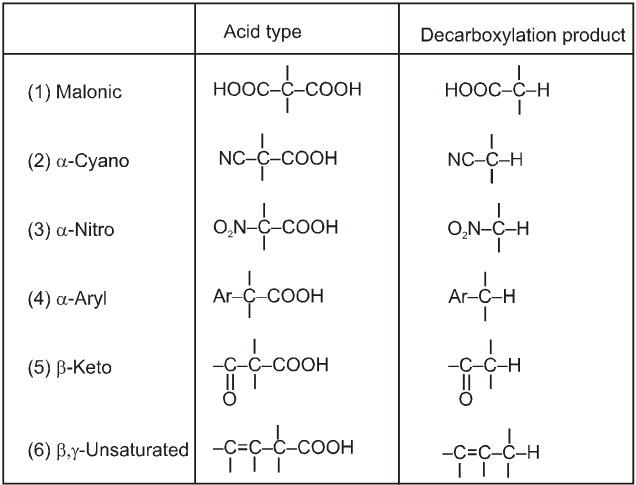
(4) Decarboxylation reactions :
(i)Soda-lime decarboxylation :
General reaction :
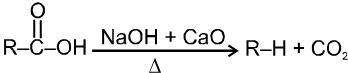
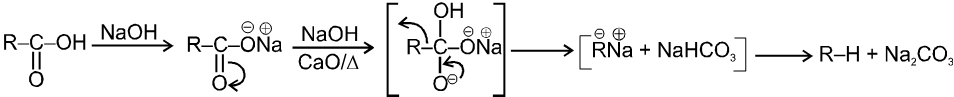
In this reaction carbanion intermediate is formed.
Rate of reaction depends upon the stability of carbanion intermediate.
Electron withdrawing group at R–COOH will increases the rate of decarboxylation.
Ex.
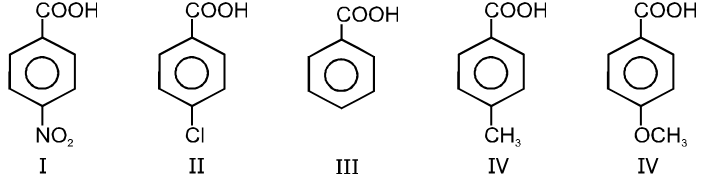
Rate of decarboxylation. I > II > III > IV > V
Ex.
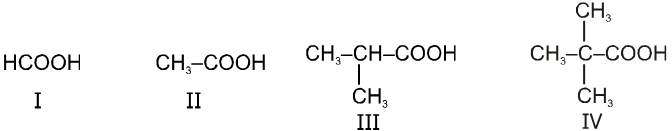
Rate of decarboxylation. I > II > III > IV
(ii) Decarboxylation of b-keto carboxylic acids:
b-keto acids decarboxylate readily when they are heated to 100–150ºC.
![]()
There are two reasons for ease of decarboxylation.
When the acid itself decarboxylates, it can do so through a six membered cyclic transition state :
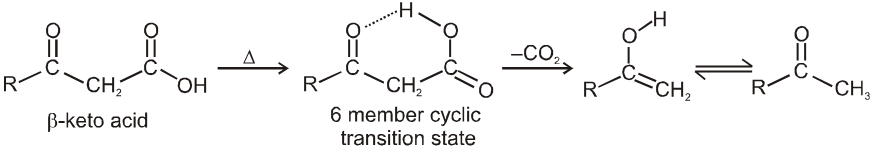
This reaction produces an enol directly and avoids an anionic intermediate. The enol then tautomerises to a methyl ketone.
When the carboxylate anion decarboxylates, it forms a resonance stabilized enolate anion.
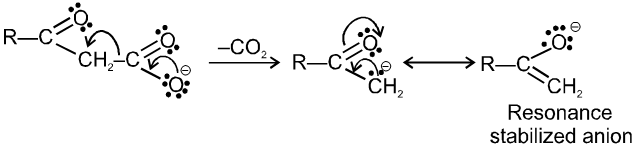
Alphatic acids that do undergo successful decarboxylation have certain functional groups or double or triple bonds in the a or b positions.
(iii) Kolbe’s electrolysis
![]()
If n is the number of carbon atoms in the salt of carboxylic acid, the alkane formed has 2(n–1) carbon atoms.
Ex.
![]()
(iv) Hunsdiecker Reaction (Bromo-decarboxylation) :
R–COOAg + Br2 ¾®R–Br + CO2 + AgBr
Heavy metal salt of acid is heated with Br2 it results in decarboxylations.
Metal can be silver ion, mercuric ion, or lead (IV) ion.
Mechanism :
Step 1 : ![]()
Step 2 : ![]()
Step3 : ![]()
Step 4 : ![]()
Although bromine is the most often used halogen, chlorine and iodine have also been used.
When iodine is the reagent, the ratio between the reactant is very important and determines the product.
A 1 : 1 ratio of salt to iodine gives alkyl halide, as above. A 2 : 1 ratio, however gives the ester RCOOR. This is called Simonini reaction and sometimes used to prepare carboxylic ester. The yield of alkyl halide follows the order Primary > Secondary > Tertiary
(5) HVZ Reaction (Halogenation of aliphatic acids and Substituted acids) :
In the presence of a small amount of phosphorus, aliphatic carboxylic acids react smoothly with chlorine or bromine to yield a compound in which -hydrogen has been replaced by halogen.
![]()
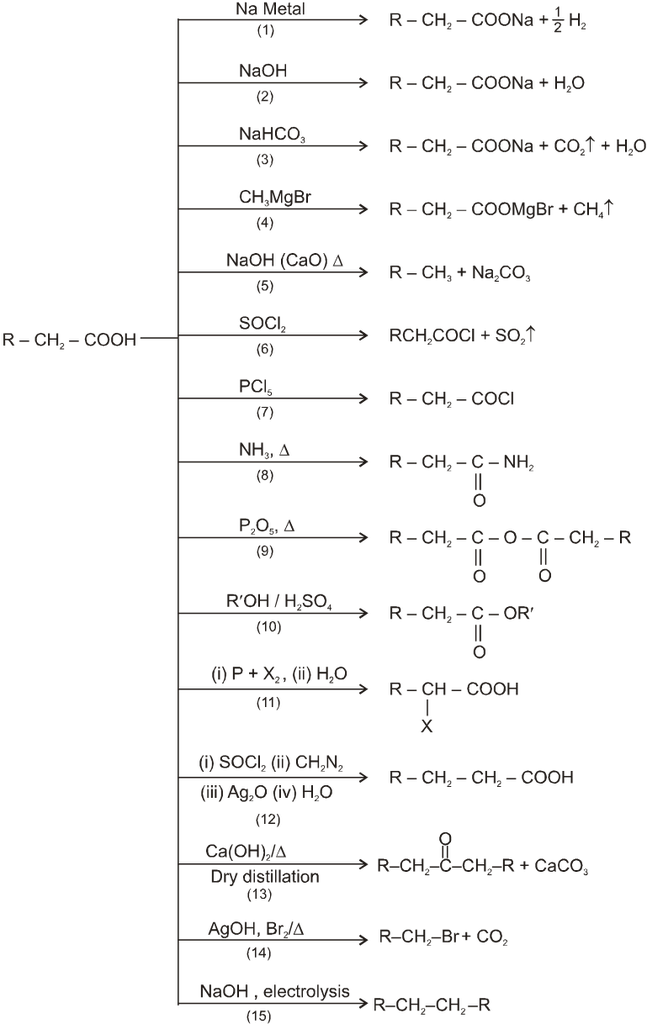
Summary of reactions of carboxylic acids :
11. Uses of Carboxylic Acids
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Uses of Carboxylic Acids
- Methanoic acid - In rubber, textile, dyeing, leather and electroplating industries.
- Ethanolic acid - As a solvent and as a vinegar in food industry.
- Hexanoic acid - In the manufacture of nylon-6, 6.
- Higher fatty acids - For the manufacture of soaps and detergents.
- Esters of benzoic acid - In perfumery.
- Sodium benzoate - As a food preservative.
12. Carboxylic Acid Derivatives
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Carboxylic Acid Derivatives
Closely related to the carboxylic acids and to each other are a number of chemical families known as functional derivatives of carboxylic acids : acid chlorides, anhydrides, amides, and esters, These derivatives are compounds in which the —OH of a carboxyl group has been replaced by —CI,—OOCR,
—NH2, or —OR`.
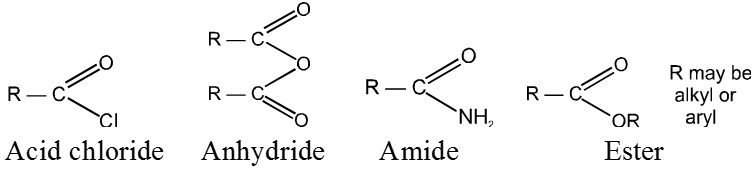
They all contain the acyl group, ![]()
Like the acid to which it is related, an acid derivative may be aliphatic or aromatic, substituted or unsubstituted; whatever the structure of the rest of the molecule, the properties of the functional group remain essentialy the same.
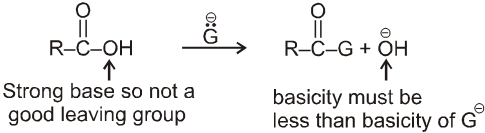
Characteristic reaction of acid deerivatives (Nucleophilic acyl substitution) :
Nucleophilic acyl substitution usually takes place by an addition-elimination mechanism.The incoming nucleophile adds to the carbonyl to form a tetrasubstituted intermediate with a tetrahedral carbon.
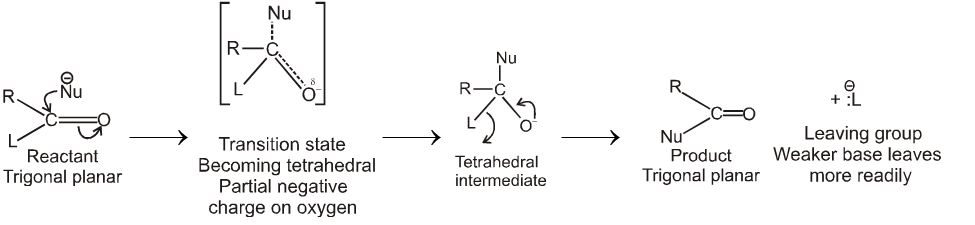
The tetrahedral intermediate formed when a nucleophile attacks the carbonyl carbon of a carboxylic acid derivative is not stable and cannot be isolated.
A pair of nonbonding electrons on the oxygen reforms the bond, and either  or
or  is eliminated with its bonding electrons. Whether
is eliminated with its bonding electrons. Whether  or
or  is eliminated depends on their relative basicities. The weaker base is preferentially eliminated because the weaker the base, the better it is a leaving group.
is eliminated depends on their relative basicities. The weaker base is preferentially eliminated because the weaker the base, the better it is a leaving group.
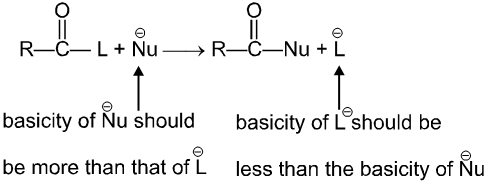
Thus carboxylic acid derivative will undergo a nucleophilic acyl substitution reaction provided that the incoming nucleophile is a stronger base than the group that is to be replaced. If the incoming nucleophile and the group attached to acyl group in the starting material have similar basicities, the tetrahedral intermediate can expect either group with similar ease. A mixture of starting material and substitution product will result.
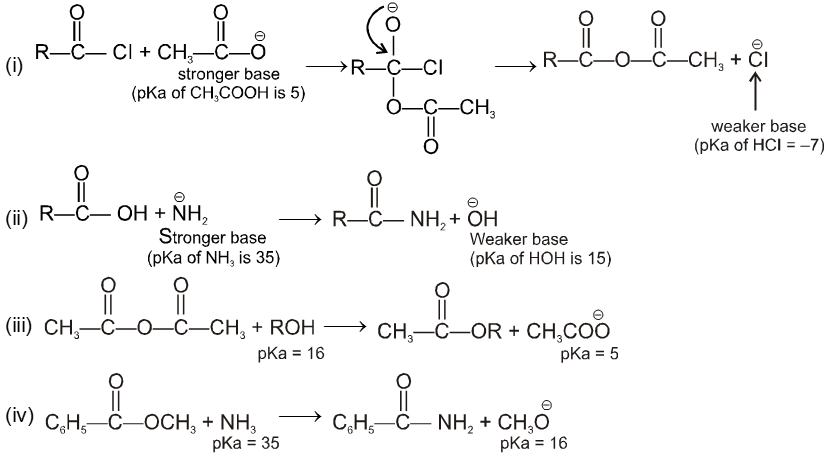
Condition for acyl nucleophilic substitution reaction :
![]()
(i) L must be better leaving group than ![]() , i.e., basicity of Nu should be more than that of
, i.e., basicity of Nu should be more than that of
(ii) ![]() must be a strong enough nucleophilic to attack RCOL.
must be a strong enough nucleophilic to attack RCOL.
(iii) Carbonyl carbon must be enough electrophilic to react with ![]() .
.
Reactivity order : 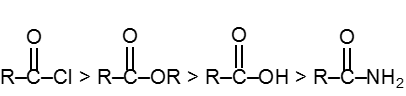
(A) Acid halides :
Methods of preparation of Acyl halides :
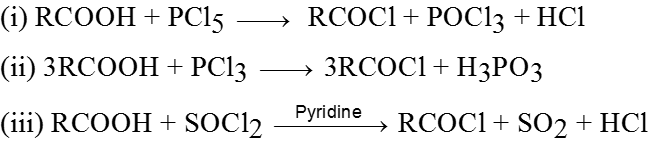
Ex. 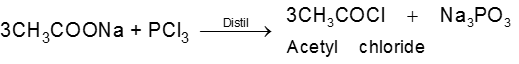
Ex. 
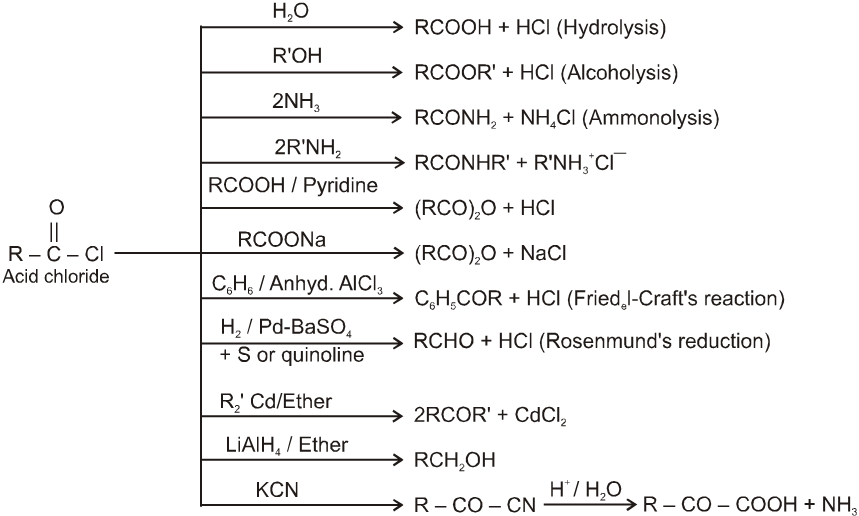
Chemical Reactions :
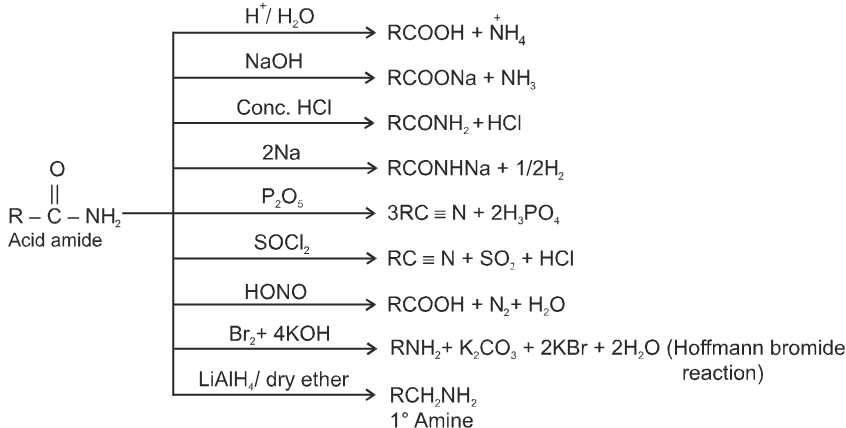
(B) Acid amides :
Methods of preparation of acids amides :
(1) By reaction of esters with ammonia and amines :
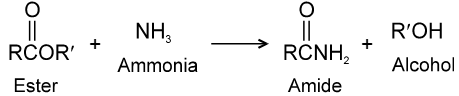
Ammonia is more nucleophilic than water, making it possible to carry out this reaction using aqueous ammonia.
(2) From acid halides :
RCOCl + 2NH3![]() RCONH2 + NH4Cl
RCONH2 + NH4Cl
(3) From anhydride :
(RCO)2O + 2NH3 ![]() RCONH2 + RCOONH4
RCONH2 + RCOONH4
(4) From ammonium salt of carboxylic acid :
RCOONH4 ![]() RCONH2 + H2O
RCONH2 + H2O
CH3COONH4 ![]() CH3CONH2
CH3CONH2
(5) From cyanides :
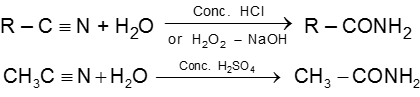
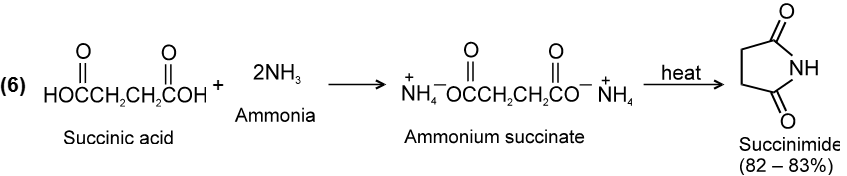
Chemical Reactions :
(C) Esters :
Methods of Preparation :
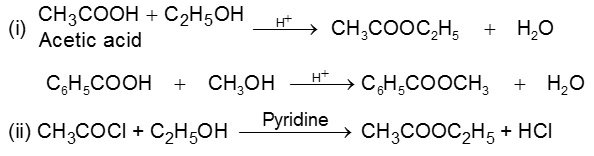
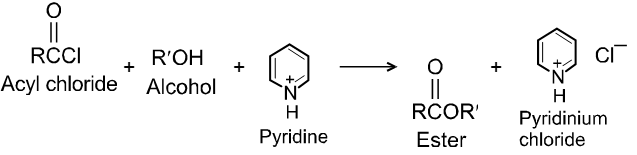
Alcohols react with acyl chlorides by nucleophilic acyl substitution to yield esters. These reactions are typically performed in the presence of a weak base such as pyridine.
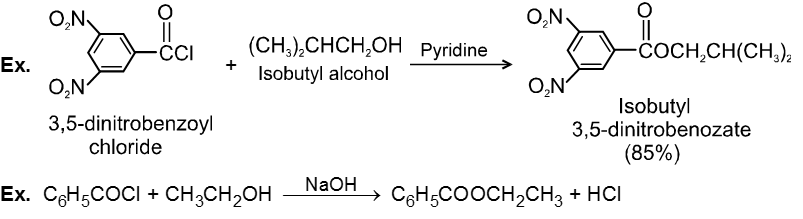
Chemical Reactions :
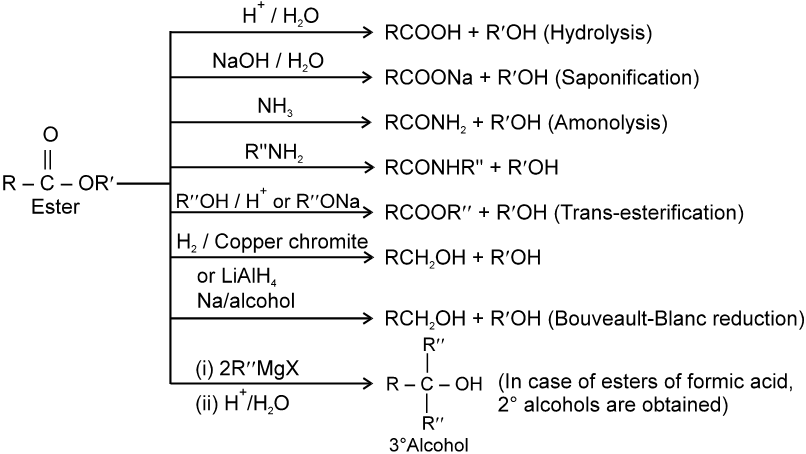
(D) Acid anhydrides :
Methods of Preparation of acid anhydrides :
(1) From carboxylic acids :
![]()
Ex. 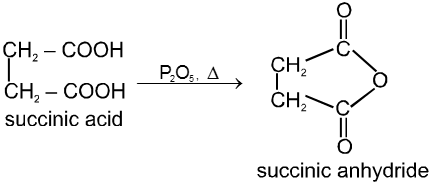
Ex.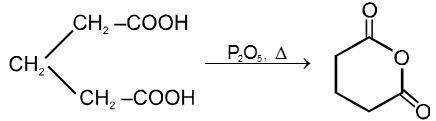
Ex. 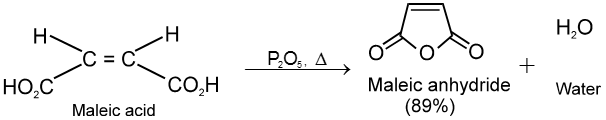
(2) From acid and acid halide :
Ex. ![]()
Ex. ![]()
Chemical Reactions
(1) Reaction with aromatic compounds (Friedel crafts acylation) :
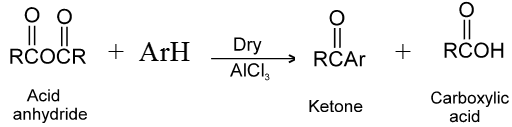
Ex.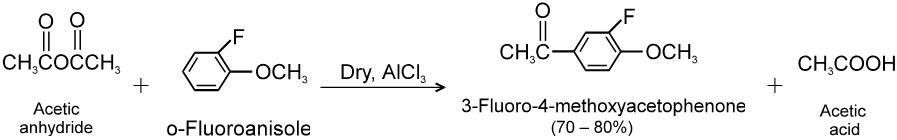
(2) Reaction with alcohols :
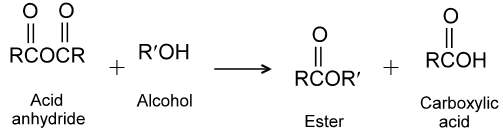
Ex.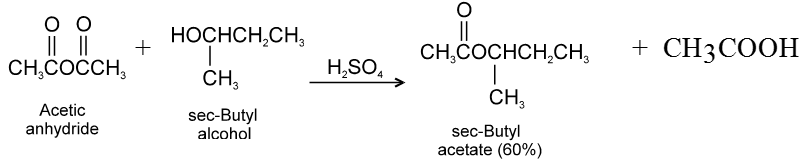
(3) Reaction with ammonia and amines :
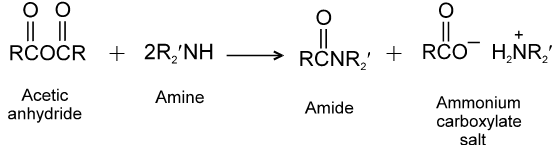
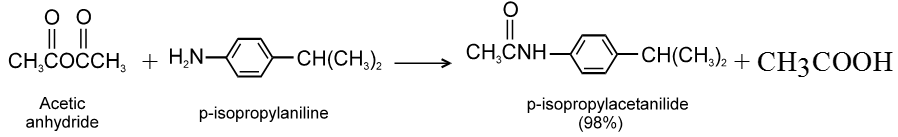
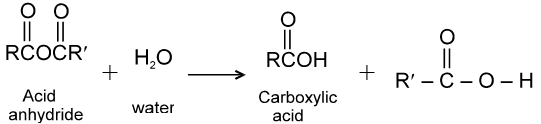
(4) Hydrolysis :
Acid anhydrides react with water to yield two carboxylic acid functions. Cyclic anhydrides yield dicarboxylic acids.
Heating Effects :
(a) Heating effect on monocarboxylic acid :
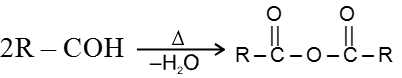
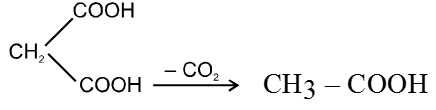
(b) Heating effect on dicarboxylic acid :
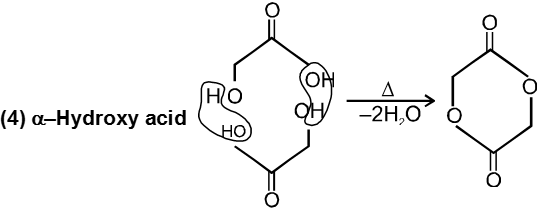
(c) Heating effects on Hydroxy acids :
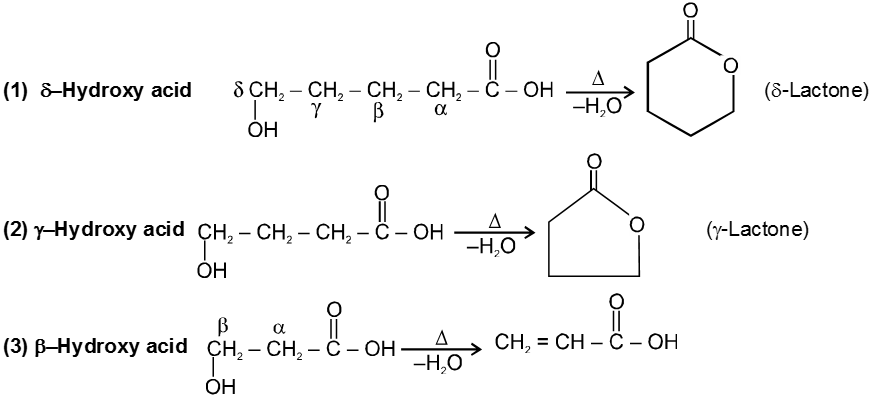
Since 4 or 8 membered rings are less stable the refore b-Hydroxy acids on heating produce a, b unsaturated carboxylic acid.
(d) Heating effects on esters :
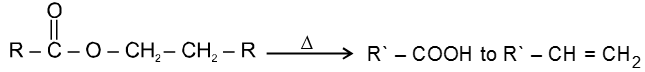
Mech :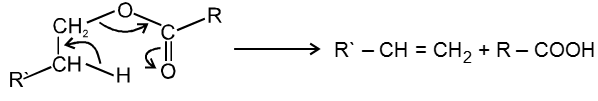
This reaction follows syn elimination & hoffman product is formed.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
