- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Bonding in coordination compounds
Valence bond theory :
The valence bond theory, VBT, was extended to coordination compounds by Linus Pauling in 1931. The formation of a complex involves reaction between a lewis base (ligand) and a lewis acid (metal or metal ion) with the formation of a coordinate-covalent (or dative) bonds between them. The model utilizes hybridisation of (n-1) d, ns, np or ns, np, nd orbitals of metal atom or ion to yield a set of equivalent orbitals of definite geometry to account for the observed structures such as octahedral, square planar and tetrahedral, and magnetic properties of complexes. The number of unpaired electrons, measured by the magnetic moment of the compounds determines which d-orbitals are used.
These hybrid orbitals are allowed to overlap with ligand orbitals that can donate electron pairs for bonding.
Following table provides the types of hybridisation with different coordination number.
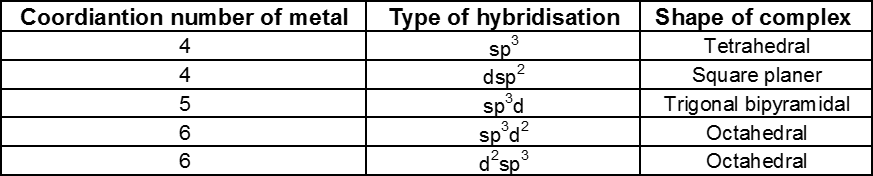
It is to be noted that the type of hybridisation of metal and shape of complex involved can be predicted conveniently, if some characteristic of the complex like magnetic nature, geometry or whether exhibits isomerism or not, etc., be known.
Coordination Number Six.
In the diamagnetic octahedral complex, [Co(NH3)6]3+, the cobalt ion is in +3 oxidation state and has the electronic configuration represented as shown below.
Co3+,[Ar]3d6 ![]()
[Co(NH3)6]3+ ![]()
![]()
Thus, the complex has octahedral geometry and is diamagnetic because of the absence of unpaired electron. Since in the formation of complex the inner d-orbital (3d) is used in hybridisation, the complex is called an inner orbital or low spin or spin paired complex.
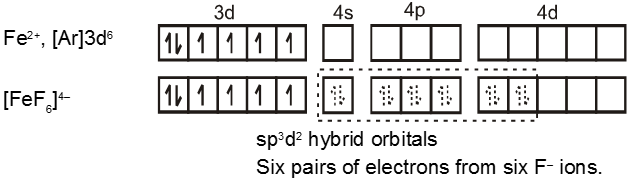
The complex [FeF6]4– is paramagnetic and uses outer orbital (4d) in hybridisation (sp3d2) ; it is thus called as outer orbital or high spin or spin free complex. So :
Coordination Number Four :
In the paramagnetic and tetrahedral complex [NiCl4]2–, the nickel is in +2 oxidation state and the ion has the electronic configuration 3d8. The hybridisation scheme is as shown in figure.
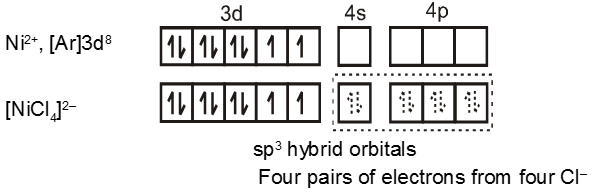
The compound is paramagnetic since it contains two unpaired electrons.
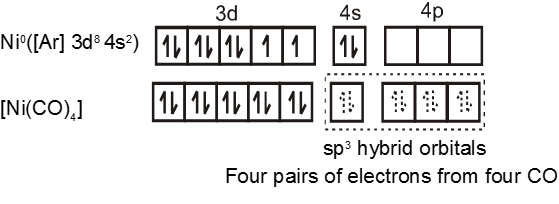
Similarly complex [Ni(CO)4] has tetrahedral geometry and is diamagnetic as it contains no unpaired electrons. The hybridisation scheme is as shown in figure.
Complexes of Pd(II) and Pt (II) are usually four-coordinate, square planar, and diamagnetic
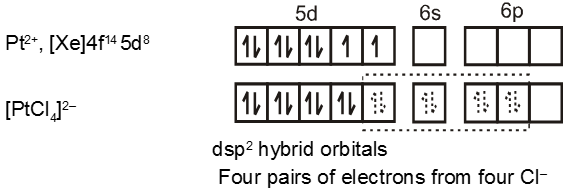
Similarly the hybridisation scheme for [Ni(CN)4]2– is as shown in figure.
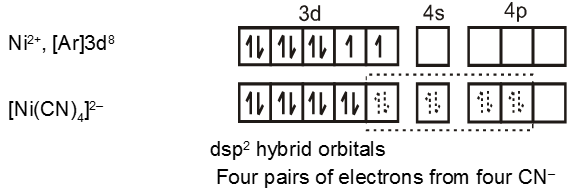
While the valence bond theory, to a large extent, explains the formation, structures and magnetic behaviour of coordination compounds, it suffers from the following shortcomings :
1. A number of assumptions are involved.
2. There is no quantitative interpretation of magnetic data.
3. It has nothing to say about the spectral (colour) properties of coordination compounds.
4. It does not give a quantitative interpretation of the thermodynamic or kinetic stabilities of coordination compounds.
5. It does not make exact predictions regarding the tetrahedral and square-planar structures of 4-coordinate complexes.
6. It does not distinguish between strong and weak ligands.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
