- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Alchols and Ethers – Introduction and Classification
Alcohols :
Alcohols have sp3 hybridized oxygen atoms, but the C – O – H bond angle in methanol (108.9°) is considerably larger than the H – O – H bond angle in water (104.5°) because the methyl group is much larger than a hydrogen atom.
The bulky methyl group counteracts the bond angle compression caused by oxygen’s nonbonding pairs of electrons. The O – H bond lengths are about the same in water and methanol (0.96 Å), but the C – O bond is considerably longer (1.4 Å), reflecting the larger covalent radius of carbon compared to hydrogen.

Classification of alcohols:
(a) Alcohols may be classified as mono–, di–, tri- or polyhydric alcohols depending on whether they contain one, two, three,.............hydroxy group.
Monohydric : Contains one –OH group (C2H5OH ; CH3–CH2–CH2–OH)
Dihydric : Contains two –OH group ![]() (Glycol)
(Glycol)
Trihydric (Polyhydric): Contains three or more than three –OH groups ![]() (Glycerol)
(Glycerol)
(b) Allylic alcohols: CH2=CH–CH2–OH ; 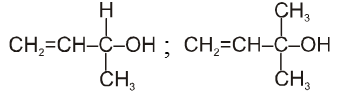 ;
;
(c) Vinylic Alcohol: CH2=CH–OH (unstable at room temperature)
(d) Benzylic alcohols:
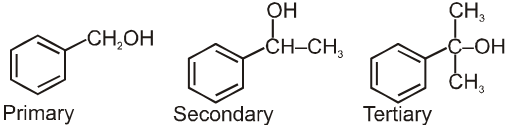
Ethers :
Like water, ethers have a bent structure, with an sp3 hybrid oxygen atom giving a nearly tetrahedral bond angle.
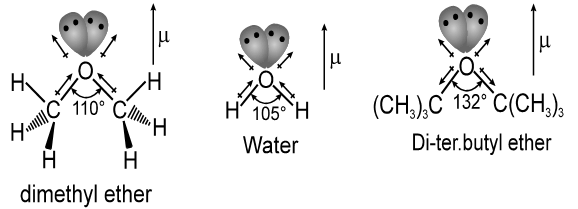
Bonding in ethers is readily understood by comparing ethers with water and alcohol. Van der Waals strain involving alkyl groups causes the bond angle at oxygen to the larger in ethers than in alcohol, and larger in alcohols than in water. An extreme example is di-tert-butyl ether, where steric hindrance between the tert-butyl groups is responsible for a dramatic increase in the C – O – C bond angle.
Classificiation of ethers :
(a) Simple or symmetrical : If the alkyl or aryl groups attached to the oxygen atom are the same.
CH3OCH3, C2H5OC2H5
(b) Mixed or unsymmetrical : If the two groups attached to the oxygen atom are different.
C2H5OCH3, C2H5OC6H5.
General Reactions of alkyl halides, alcohols & ethers :


 ACME SMART PUBLICATION
ACME SMART PUBLICATION
