- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Physical properties of organic compounds
1. Bond Polarity :
A bond with electrons shared equally between the two atoms is called a nonpolar covalent bond.
Ex. Bond in H2 and the C–C bond in ethane are nonpolar covalent bonds.
Generally in most of the bonds between two different elements, the bonding electrons are attracted more strongly towards one of the two nuclei. An unequally shared pair of bonding electrons is called a polar covalent bond.
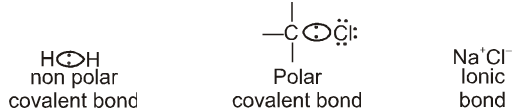
A covalent bond will be polar if atoms forming it differ in electronegativity.
Electronegativity F > O > Cl , N > Br > C,H
2. Intermolecular forces :
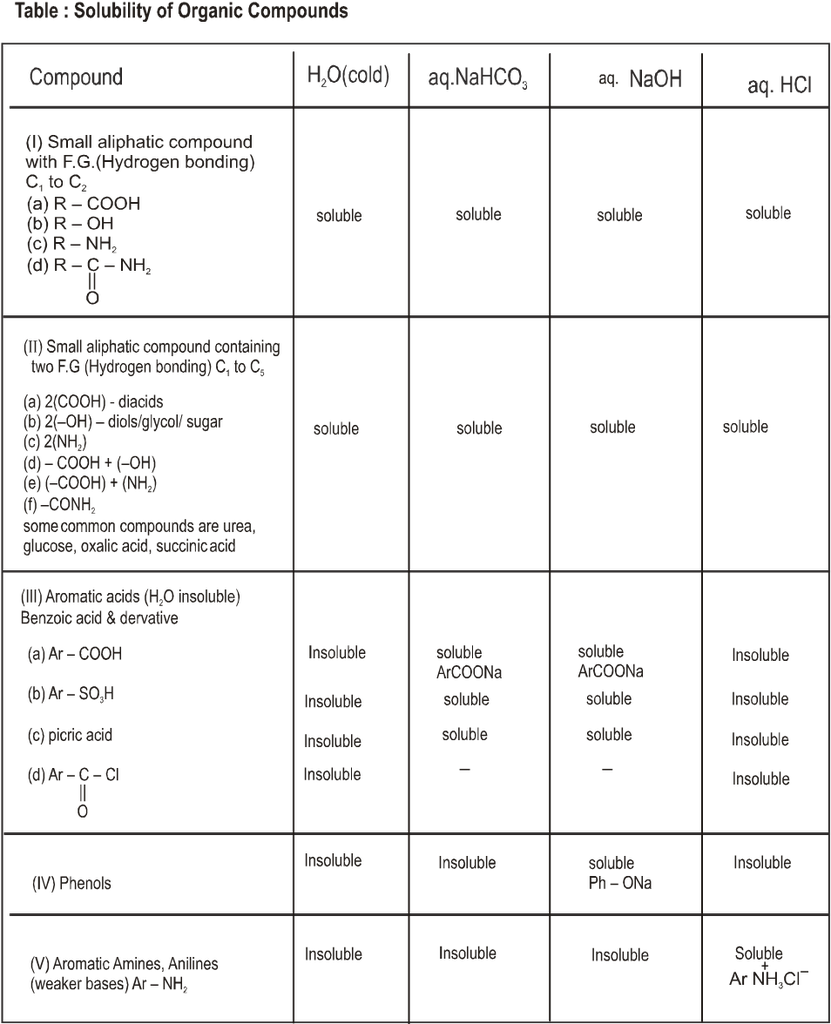
• Attractions between molecules are particularly important in solids and liquids. In these “Condensed” phases, the molecules are continously in contact with each other. The melting points, boiling points, and solubilities of organic compounds show the effects of these forces. Two major kinds of attractive forces cause molecules to associate into solids and liquids;
(A) Dipole-dipole interactions (B) VanderWaals forces
(A) Dipole-Dipole interaction :
Dipole-dipole interactions result from the approach of two polar molecules.
• If their positive and negative ends approach, the interaction is an attractive one.
• If two negative ends or two positive ends approach, the interaction is repulsive one.
• In a liquid or a solid the molecules are mostly oriented with the positive and negative ends together and the net forces is attractive.
Symbolized by :
![]()
• An especially strong kind of dipole- dipole attraction is hydrogen bonding
Types of Hydrogen Bonding :
(a) Intermolecular hydrogen bonding : In such type of linkages the two or more than two molecules of the same compound combine together to give a polymeric aggregate.
This phenomena is also known as association.
Example :
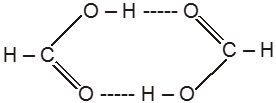
(I) Hydrogen bonding in carboxylic acids e.g. formic acid (Dimerisation)
(II) In m-Chlorophenol
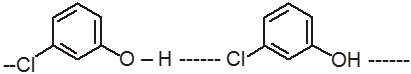
(III) In water
![]()
(IV) In p-Nitrophenol
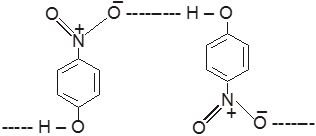
(V) In p-Nitrophenol & water
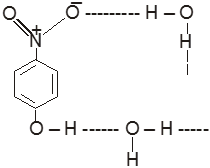
(b) Intramolecular hydrogen bonding:- In this type, hydrogen bonding occur within two atoms of the same molecule. This type of hydrogen bonding is commonly known as chelation.
Ex.
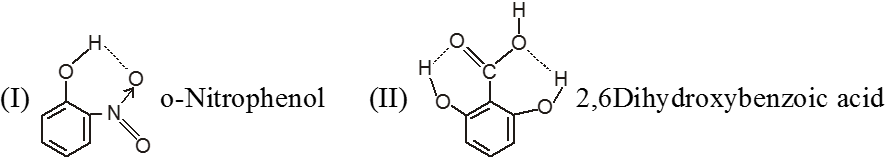
Conclusion :
(a) The chelation between the ortho substituted groups restricts the possibility of intermolecular hydrogen bonding.
(b) Chelation does not take place in m – & p – isomers because the two groups far away from each other.
3. Boiling Points :
• The boiling point (bp) of a compound is the temperature at which the compound’s vapor pressure equals the atmospheric pressure. In order for a compound to vaporize, the force that hold the molecules close to each other in the liquid must overcome. This means that the boiling point of a compound depends on the strength of the attractive forces between the individual molecules.
• If the molecules are held together by strong forces, it will take a lot of energy to pull the molecules away from each other and the compound will have a high boiling point.
• If however, the molecule are held together by weak forces, only a small amount of energy will be needed to pull the molecules away from each other and the compound will have a low boiling point.
Factors affecting boiling point :
(1) Hydrogen Bonding :
Alcohols :
Alcohols have much higher boiling points than alkanes or ethers of comparable molecular weight because, in addition to van der Waals forces and the dipole–dipole interactions of the carbon–oxygen bond alcohols can form hydrogen bonds.
The successive replacement of hydrogen atom of the –OH group of alcohol by alkyl group to form ether blocks the probability of hydrogen bonding reduces and thus B.P. of alcohols are higher than ether.
Ex. 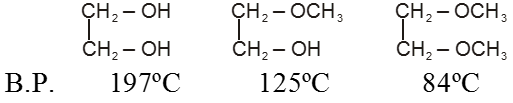
Water :
Water has the lowest molecular weight among hydrides of the VI group of periodic table, it has the highest boiling point. Water molecules associate through intermolecular hydrogen bonding and thus require more energy to separate the molecules for vaporization.
Ex. ![]()
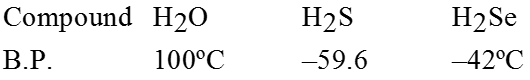
Amines :
• Primary and secondary amines also form hydrogen bonds, so these amines have higher boiling points than alkanes with similar molecular weights.
• Nitrogen is not as electronegative as oxygen, however, which means that the hydrogen bonds between amine molecules are weaker than the hydrogen bonds between alcohol molecules.
• Amines, therefore, have lower boiling points than alcohols with similar molecular weights.
• Because primary amines have two N–H bonds, hydrogen bonding is more significant for primary amines than for secondary amines. Tertiary amines cannot form hydrogen bonds with each other because they do not have a hydrogen attached to the nitrogen. Consequently if you compare amines with the same molecular weight and similar structures, primary amines have higher boiling point than secondary amines and secondary amines have higher boiling points than tertiary amines.
Ex.
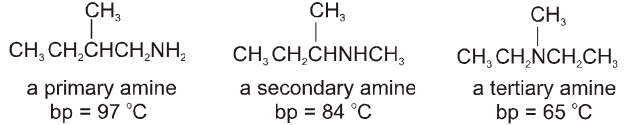
(2) Dipole - Dipole interactions :
Dipole–dipole interactions, are stronger than van der Waals forces but not as strong as ionic or covalent bonds. Ethers generally have higher boiling points than alkanes of comparable molecular weight because both van der Waals forces and dipole–dipole interactions must be overcome for an ether to boil.
Ex.

Ex. ![]()
(3) Molecular weight :
The boiling points for any homologous series of compounds increase as their molecular weights increase because of the increase in van der Waals forces. So the boiling points of a homlogous series of ethers, alkyl halides, alcohols, and amines increase with increasing molecular weight.
Ex. B. P : CH3 I > CH3Br > CH3 – Cl > CH3 – F
Ex..
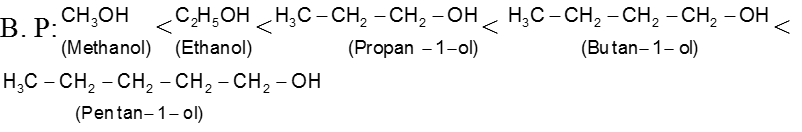
Ex.
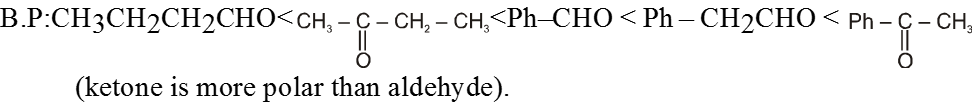
(4) Vander Waals forces
• The molecules of an alkane are held together by these induced dipole-induced dipole interactions known as van der Waals forces or London forces. In order for an alkane to boil, these van der Waals forces must be overcome.
• The homologous series of alkanes boiling points increase as their size increases. This occurs becuase each additional methylene group increases the area of contact between the molecules.
• Because the strength of van der Waals force depends on the area of contact between molecules, branching in a compound lowers its boiling point because it reduces the area of contact.
• If two alkanes have the same molecular weight, the more highly branched alkane will have a lower boiling point.
Ex.
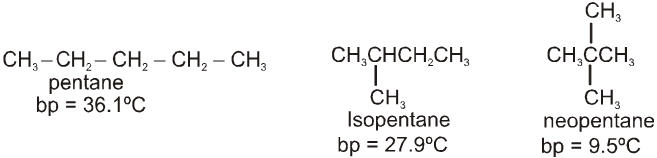
General order of boiling point of various F.G.
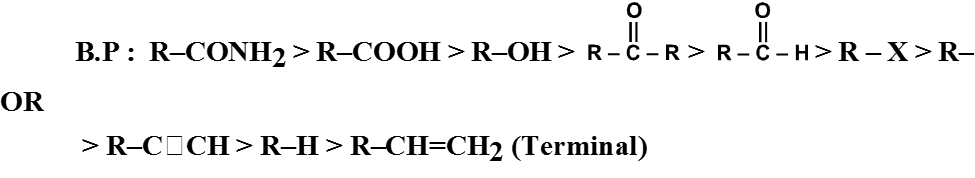
Ex. o-Chlorophenol has lower boiling point in comparison to its p-isomer.
![]() (Intramolecular H-bond in o-Chlorophenol)
(Intramolecular H-bond in o-Chlorophenol)
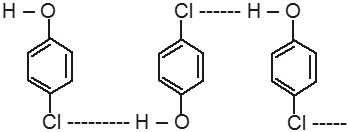 (Intermolecular H-bond in p-chlorophenol)
(Intermolecular H-bond in p-chlorophenol)
4. Melting Point :
The temperature at which the thermal energy of the particles is great enough to overcome the intracrystalline forces that hold them in position is known as melting point.
• Melting is the change from the highly orderd arrangment of particles in the crystalline lattice to the more random arrangment that characterizes a liquid.
Factors affecting melting point :
(1) Molecular weight :
Melting points of alkanes increase in a homologous series as the molecular weight increases.
Ex. M.P. : CH3–CH2–CH3 < CH3CH2–CH2–CH3 < CH3–CH2–CH2–CH2–CH3
(2) Packing :
• Packing is a property that determines how well the individual molecules in a solid fit together in the crystal lattice. If molecules are more closely packed then more energy is required to break the lattice and melt the compound.
• Alkanes with an odd number of carbon atoms pack less tightly than alkanes with an even number of carbon atoms. This decreases the intermolecular forces between alkanes with odd number of carbon atoms, which decreases their melting points.
• In geometrical isomers the trans isomers are more symmetrical than cis isomers (Cab=Cab type alkenes)
so trans form have higher M.P. than cis isomers.
- The heavier the molecule and stronger the intermolecular forces, higher will be the M.P. of the compound.
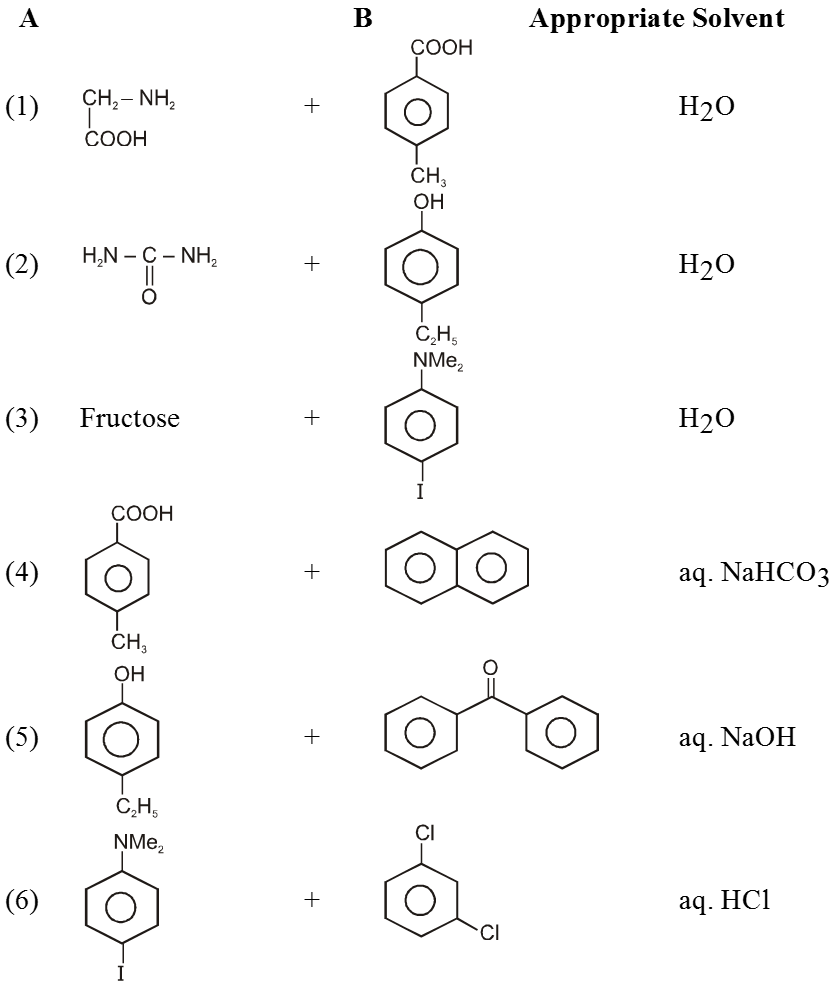
- Ortho isomers of hydroxy, nitro, carbonyl compounds have low M.P. than their corresponding m – & p – isomers.
Ex.
![]()
Ex. Ortho - hydroxy, nitro-, carbonyl, carboxylic or chloro compounds have lower melting and boiling points than the respective meta or para isomer due to interamolecular H-bonding in ortho substituted compound.
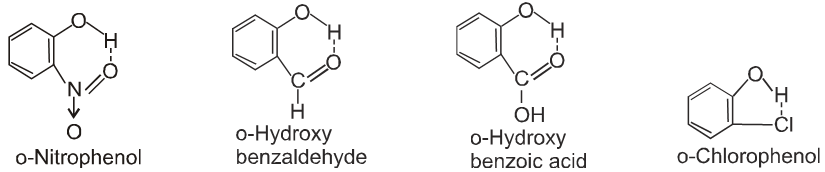
Ex. 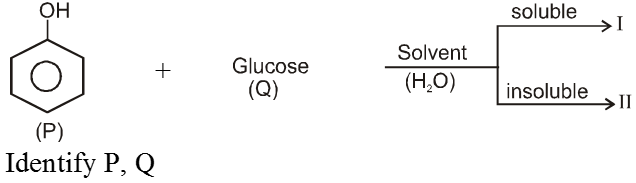
Sol. ![]()
Ex. Binary mixtures - (Two components

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
