- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Types of Reagents :
Reagents are of two types :
(i) Electrophiles
(ii) Nucleophiles
Electrophiles :
Electrophiles are electron deficient species.
Ex. ![]() (positively charged species),
(positively charged species),
![]() (species with vacant orbital at central atom).
(species with vacant orbital at central atom).
Nucleophiles and their nucleophilicity :
Nucleophile is a species having negative charge or lone pair of electrons.
They are electron rich species.
Ex. ![]() (l.p on O-atom),
(l.p on O-atom), ![]() (negaively charged species)
(negaively charged species)
Note : :CCl2 is not a nucleophile because it is electron deficient species and act as electrophile.
- Negative ions have more nucleophilic than their neutral species
![]()
- Down the group nucleophilicity increases because the more polarizable donar atom is better nucleophyle
Polarizability size of donar atom
![]()
- Across the period nucleophilicity decreases
![]()
- Bulky base has less nucleophilic character.
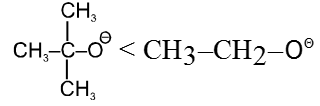
- Effect of solvent : In case of polar aprotic solvents nucleophilicity order of halides is just reversed.
Bases and their basicity :
Bases are the species which accept the proton or which donates l.p. of electron to proton.
- Basicity decreases down the group while nucleophilicity increases
F– > Cl– > Br– > I–
- Nucleophilicity and basicity order will be same across the period.
- For the same donor atom nucleophilicity and basicity order will be same
Leaving group ability :
- Weaker base is better leaving group.
- More resonance stabilised ion will be better leaving group.
- Weaker the carbon-leaving group bond (C–X) better will be the leaving group.
- If activation energy of a reaction is low then reaction will be fast and leaving group will be better.
Ex. (a) I– > Br– > Cl– > F–
(b) CF3SO3– > RCOO– > C6H5O– > OH– >
(c) 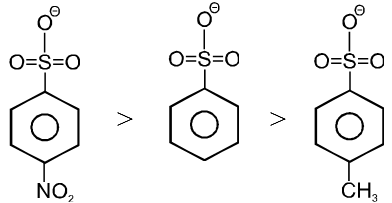
Note : More stable anions are weak bases & hence better leaving group.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
