- Books Name
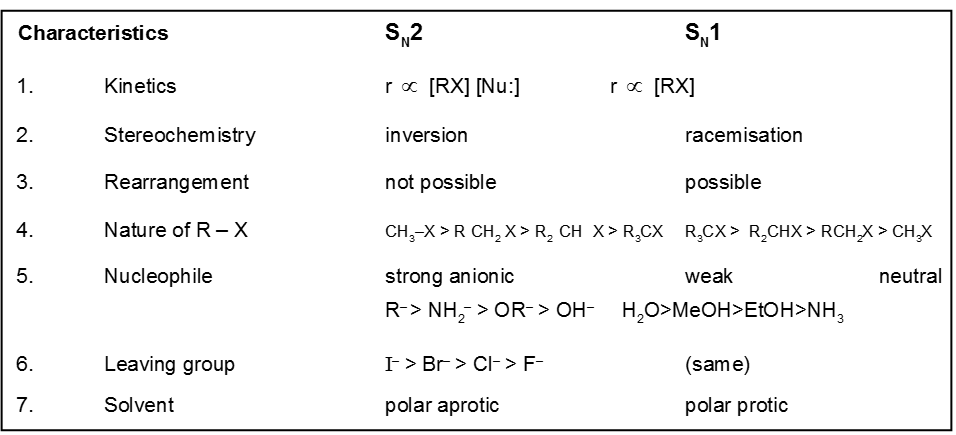
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Nucleophilic substitution reactions of alkyl halides
Alkyl halide undergoes nucleophilic substitution reaction.
![]()
Nucleophilic substitution reactions are of two types :
Unimolecular nucleophilic substitution reaction (SN1) :
- It is a two step process.
- First step is the heterolytic cleavage of carbon halogen bond (
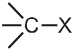 ) to give carbocation intermediate.
) to give carbocation intermediate.
![]() (Ist step is r.d.s.)
(Ist step is r.d.s.)
- In the second step nucleophile attacks from either side of carbocation to generate product (racemic mixture).
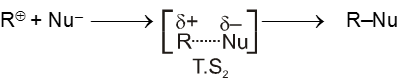 (IInd is fast step)
(IInd is fast step)
Mechanism :
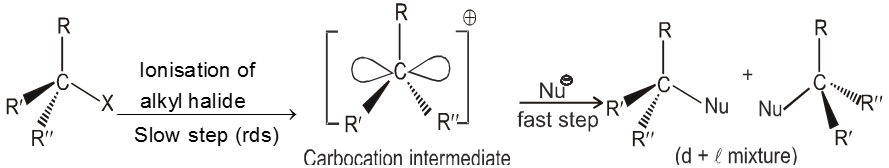
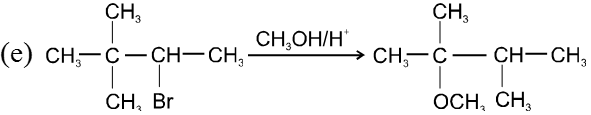
- Carbocation intermediate is formed so rearrangement is possible in SN1 reaction.
Kinetics :
- Rateµ [Alkyl halide]
- It is unimolecular and first order reaction.
- Rate of SN1 reaction is independent of the concentration and reactivity of nucleophile.
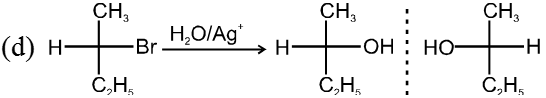
Stereochemistry :
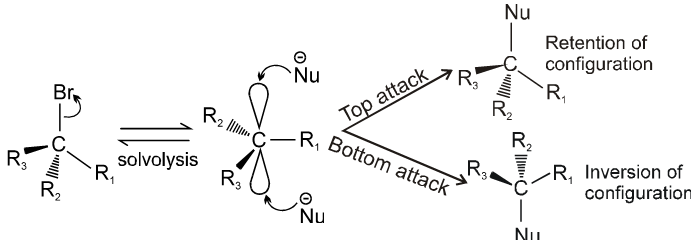
- Catalyst used is Ag+.
- More polar protic solvent is more favourable for SN1.
H2O > ROH > NH3 (order of polar protic solvent).
- In SN1 reaction carbocation is formed along with anion and to solvate these ions, polar protic solvent is used.
- Decreasing order of reactivity of alkyl halides. ;
R–I > R–Br > R–Cl > R–F
Ex.
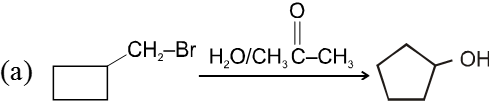
![]()
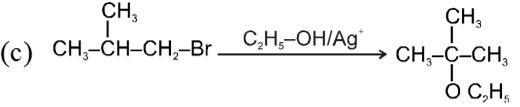
Bimolecular nucleophilic substitution reaction (SN2) :
It is a single step reaction as the rate of reaction depends upon concentration of substrate as well as nucleophile.
Mechanism :
- In this reaction nucleophile attack from back side on the carbon atom bearing leaving group. it is a concerted reaction where bond breaking and bond formation takes place simultaneously to achieve a transition state (trigonal bipyramidal shape) where half bond has been formed and half bond has been broken.
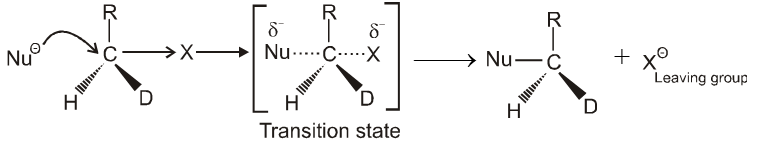
Kinetics :
- rateµ [alkyl halide] [nucleophile]
- It is a bimolecular, one step concerted process.
- It is a second order reaction because in the r.d.s. both species are involved.
Steriochemistry :
Only one product is formed where inversion of configuration takes place.
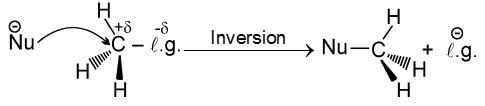
- Polar aprotic solvent is favourable not polar protic solvent. Becuase in case of polar protic solvent nucleophilicity of anion is decreased due to solvation and such solvation is not possible in case of polar aprotic solvent.
- Electron withdrawing group increases the rate of sN2 reaction.
O=CH–CH2–Br > CH3–O–CH2–Br > H–CH2–Br > CH3–CH2–Br
Comparision between SN1 / SN2 reaction :
Examples of SN2 reaction of alkyl halides :
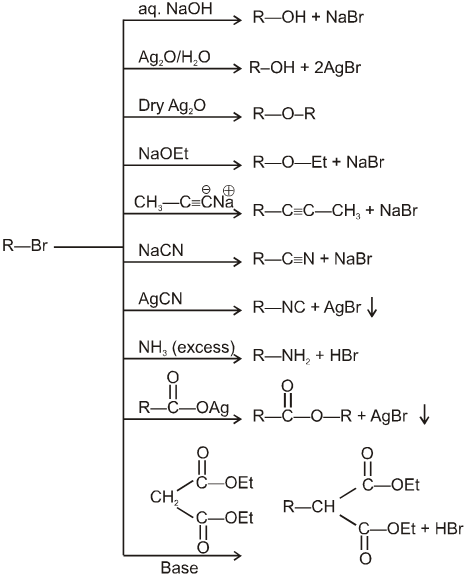
(i) Williamson’s synthesis of ethers :
- It is used for the preparation of symmetrical as well as unsymmetrical ether.
R—Br + NaOEt ¾¾®R—OEt + NaBr
- Williamson’s synthesis involve attack of an alkoxide on alkyl halide to give ethers. In place of alkoxide, phenoxide can also be used.
- This is an SN2 reaction because alkoxide is a strong nucleophile.
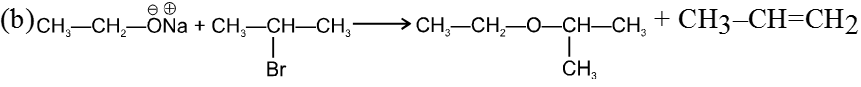
- On using 2º & 3º alkylhalide we get alkene not ether as a product.
Ex.
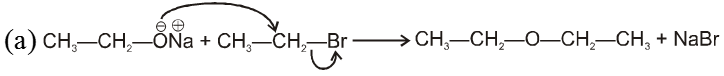
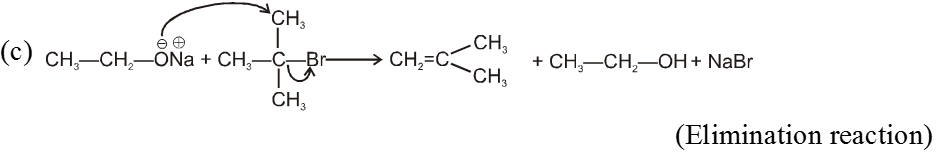
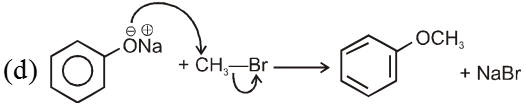
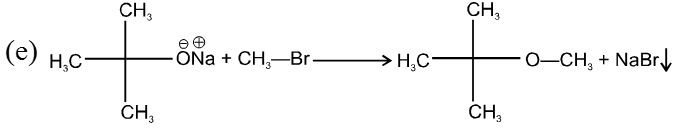
Note : Vinyl or Aryl halide should not be used because they don’t give SN2 reaction.
Ex. 
(ii) Reaction with AgCN and NaCN :
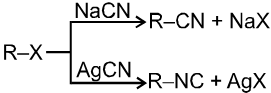
- NaCN is a more ionic hence ionized to give free
 (an ambident nucleophile) where carbon side is more active than nitrogen side. That is why with NaCN, RCN is formed.
(an ambident nucleophile) where carbon side is more active than nitrogen side. That is why with NaCN, RCN is formed.
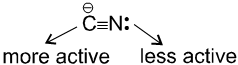
- On the other hand AgCN is more covalent so it is not ionized therefore only nitrogen side is free to act as a nucleophile and give isocyanide (R—NC)
(iii) Reaction with NH3 :

![]()
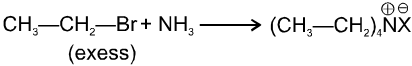
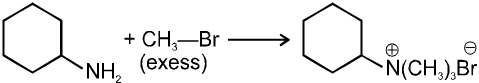
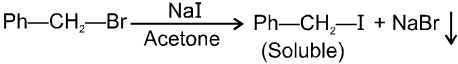
(iv) Finkelstein reaction :
R—OH + HI¾¾®R—I ![]() R—H + I2 (reduced) [Final product is Alkane (R-H) not R-I]
R—H + I2 (reduced) [Final product is Alkane (R-H) not R-I]
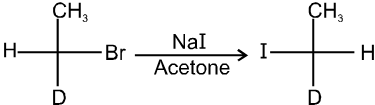
This is a problems that is why iodides are best prepared through halogen exchange reaction. It is also known as Finkelstein reaction. In this reaction R—Cl and R—Br is treated with sodium iodide in acetone.
![]()
- NaI is soluble in acetone. In this reaction acetone is used because sodium iodide is soluble in acetone but NaBr and NaCl are insoluble so precipitated out. This eliminates any possibility of reverse reaction.
- It is an SN2 reaction therefore only 1ºRX and 2ºRX is used.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
