- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
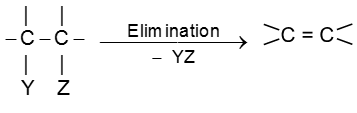
Elimination reactions of alkyl halides
In an elimination reaction two atoms or groups (YZ) are removed from the substrate and generally resulting into formation of p bond.
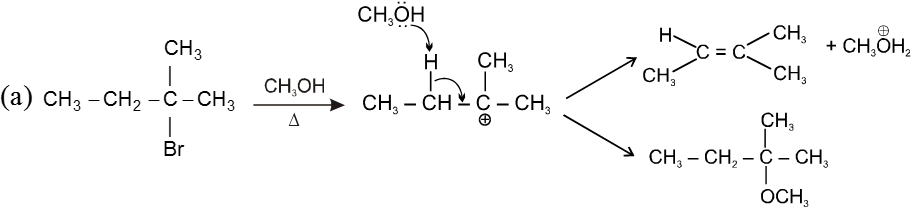
Unimolecular elimination reaction (E1) :
Proton and leaving group depart in two different step.
Mechanism :
Step 1 : Formation of the carbocation (r.d.s.)

Step 2 : Base ( ) abstracts a proton (fast step)

- Reaction intermediate is carbocation, so rearrangment is possible
Kinetics:
- Rateµ[Alkylhalide]
- It is a unimolecular and first order reaction.
- Reactivity order is similar to SN1 becuase Carbocation Intermediate is formed in the rds step.
SN1 v/s E1 :
- In case of alkyl halides SN1 product is generally more than E1 product
Ex.
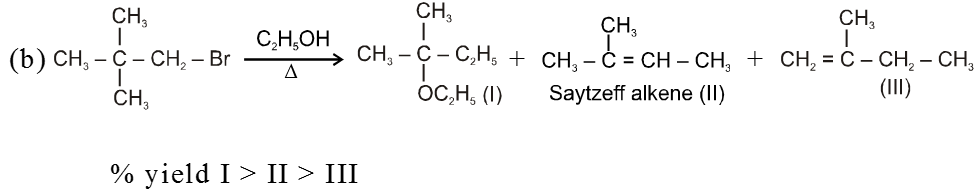
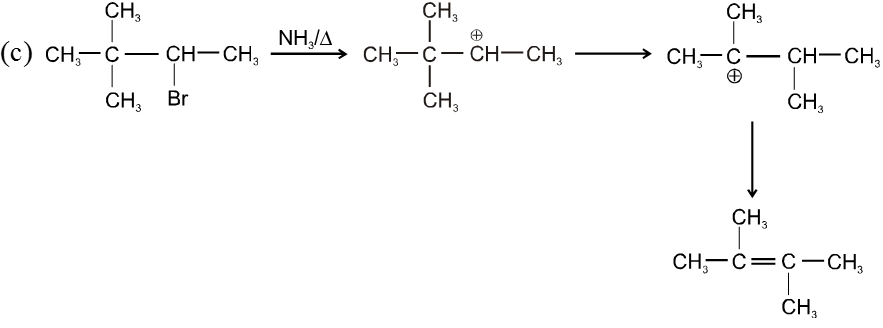
Bimolecular elimination reaction (E2) :
- It is a second order reaction because rate of reaction depends upon conc. of substrate as well as base.
Mechanism :
- Orientation of eliminated proton and leaving group are antiparallel or antiperiplanar to each other because in anti conformation the transition state is more stable due to minimum electronic repulsion.
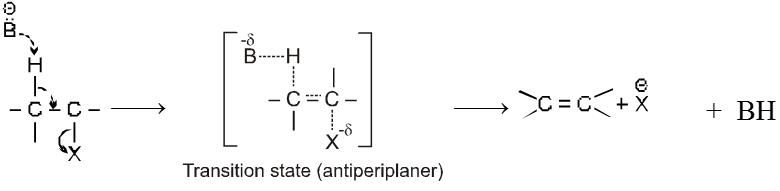
- E2 reaction is stereospecific.
- E2 reaction also depends upon size of base which decides major or minor product.
Kinetics :
- Rate µ [R – X] [Base]
- This is a single step, bimolecular reaction
- No carbocation intermediate is formed hence there is no rearrangement but a transition state is achieved because it is a single step reaction.
- More favourable substrate is tertiary alkyl halide because it will give more stable alkene according to saytzeff rule.
Ex.
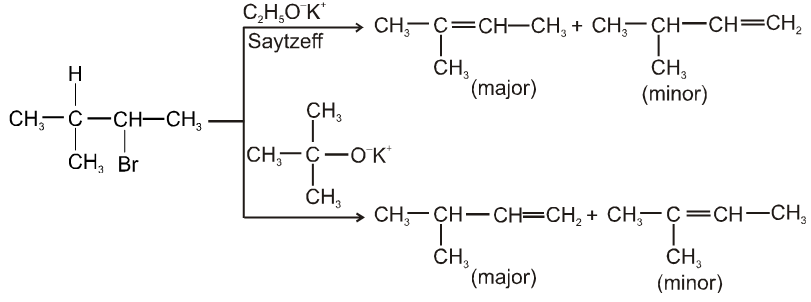
Some important terms :
(a) Regioselective reaction : Those reaction in which more than one structural isomeric products are possible, said to be regioselective reaction.
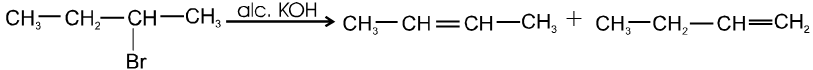
(b) Regiospecific reaction : Those reaction in which only one structural isomer product is formed out of the possible products, said to be regiospecific reaction.
(c) Stereoselective : Those reactions in which mixture of two stereoisomeric products are formed with one major product.
Examples : SN1, SN2 and E2
(d) Stereospecific reaction : Those reactions in which two stereoisomeric reactants give two different stereoisomeric products, are called as stereospecific reactions.
Examples : SN2 and E2
Note : All stereospecific reactions are stereo selective.
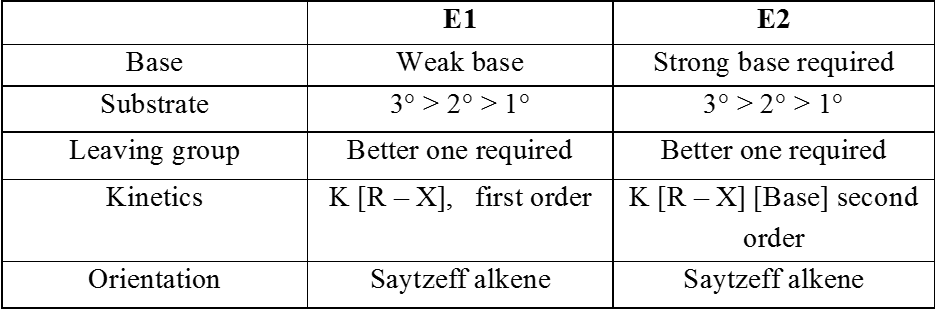
Comparison between E1 and E2 reactions :
E1cB Reaction :
- It is two step reaction.
- In first step base takes proton from adjacent carbon atom of halogen bearing carbon and generate carbanion intermediate.
-
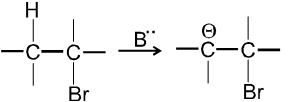
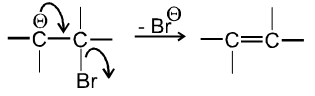
- In second step there is loss of leaving group by carbanion to get alkene :
Ex.
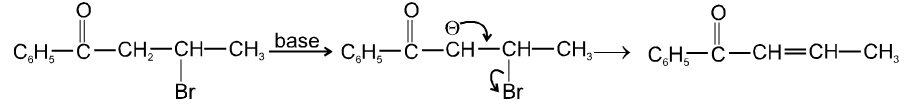
- b-hydrogen atom should be more acidic which is possible only if carbon atom having b-hydrogen atom should be link to with electron withdrawing group (-m, -I group).
- Leaving group should be more electronegative because it also increases acidity of b-hydrogen atom.
- Experimentally it is found that Ist step i.e. formation of carbanion intermediate is fast step and second step i.e. removal of leaving group is slow step thus r.d.s. in E1cB is second step.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
