- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Colour in Coordination Compounds :
According to the crystal field theory the colour is due to the d-d transition of electron under the influence of ligands. The mechanism of light absorption in coordination compounds is that photons of appropriate energy can excite the coordination entity from its ground state to an excited state. Consider the Ti(III) ion in solution, that is [Ti(H2O)6]3+. This is a violet colour octahedral complex, where in the ground state of the complex a single electron is present in t2g level. The next higher state available for the transition is the empty eg level. If the light corresponding to the energy of yellow-green is absorbed by the complex, it would excite the electron from t2g level to eg level. Consequently the complex appears violet in colour.
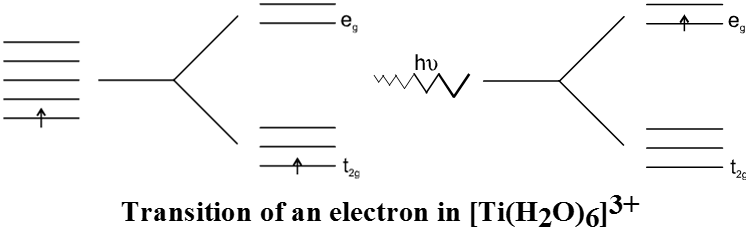
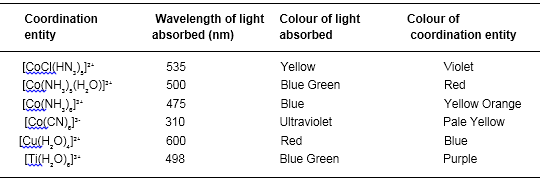
Table below gives the relationship of the wavelength absorbed and the colour observed.
Relationship between the wavelength of light absorbed and the colour observed In some coordination entitles
Note :
(a) In absence of ligand, crystal field splitting does not occur and as a consequence the substance appears colourless. For example (i) removal of water from violet coloured complex [Ti(H2O)6]Cl3 on heating makes it colourless. (ii) Similarly anhydrous copper sulphate (CuSO4) is white, but hydrated copper sulphate (CuSO4.5H2O) is blue coloured.
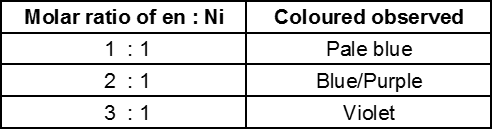
(b) The nature of the ligand and the molar ratio of metal : ligand also influence the colour of the complex for example in the pale green complex of [Ni(H2O)6], the colour change is absorbed when ethylenediamine is progressively added to it.
Note : Ruby is Al2O3 in which 0.5–1% Cr3+ ions (d3 electron system) are randomly distributed in the positions normally occupied by Al3+. We may consider Cr(III) species as octahedral Cr(III) complexes incorporated into the alumina lattice ; d-d transition of electron at these centres give rise to the colour (red).
Emerland is the mineral beryl (Be3Al2Si6O18) in which Cr3+ ions occupy octahedral sites, but in this case low energy corresponding to yellow red and blue is absorbed and light corresponding to green region is transmitted.
Limitations of crystal field theory
(1) It considers only the metal ion d-orbitals and gives no consideration at all to other metal orbitals (such as s, px, py and pz orbitals).
(2) It is unable to account satisfactorily for the relative strengths of ligands. For example it gives no explanation as to why H2O is a stronger ligand than OH– in the spectrochemical series.
(3) Account to this theory, the bond between the metal and ligands are purely ionic. It gives no account on the partly covalent nature of the metal ligand bonds.
(4) The CFT cannot account for the p-bonding in complexes.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
