- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
ANILINE (C6H5NH2)
General Methods of Preparation
(1) Lab method :
![]()
(2) Industrial method :
![]()
(3) From phenol :
![]()
(4) From benzamide (Hoffmann's bromamide reaction) :
C6H5CONH2 + Br2 + 4KOH ¾® C6H5NH2 + K2CO3 + 2KBr + 2H2O
(5) Form benzoic acid (Schmidt reaction) :
![]()
(Hydrazoic acid)
(6) From Grignard reagent :
![]()
(7) From phenyl isocyanide :
![]()
Physical properties
(i) Fresh, aniline is a colorless oily liquid. On standing the colour becomes dark brown due to action of air and light.
(ii) It's B.P. is 183ºC.
(iii) It is heavier than water.
(iv) It has characteristic unpleasent odour. It is toxic in nature.
Reactions due to –NH2 group
(1) Basis nature : Aniline is weak base but it forms salt with strong acids. It accepts a proton.
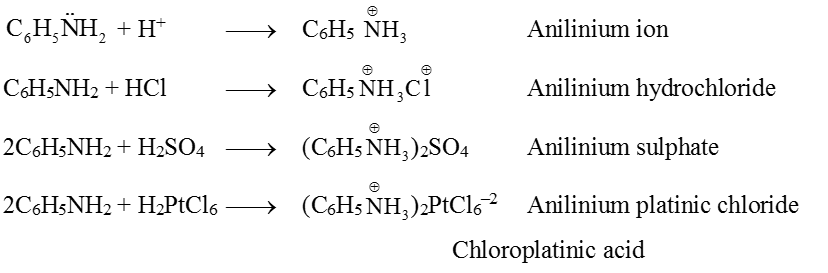
(2) Alkylation : Aniline reacts with alkyl halides forming secondary, tertiary and quaternary ammonium salts depending on the concentration of alkyl halides.
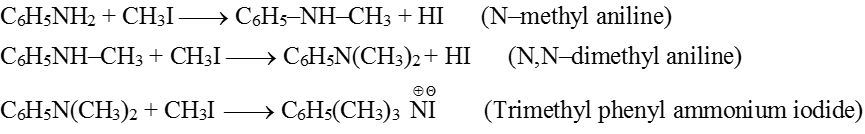
(3) Acylation : Aniline reacts with acid chlorides or anhydrides to form corresponding amides called anilides. [The reaction of C6H5NH2 with benzoyl chloride is example of "Schotten Baumann reaction"]
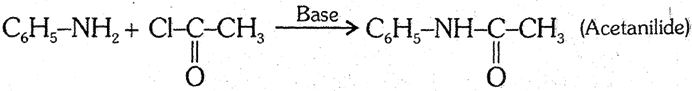
(4) Carbylamine reaction :
C6H5NH2 + CHCl3 + 3KOH ¾®C6H5NC + 3KCl + 3H2O
Phenyl isocyanide (Foul smell compound)
Note :
(1) Intermediate species is dichloro carbene [: CCl2].
(2) This is test of aniline and other primary amine, known as Isocynide test.
(5) Hoffmann's mustard oil reaction : When aniline is heated with alc. CS2 and excess of HgCl2 Phenyl isothiocyanate having a characteristic smell of mustard oil is formed.
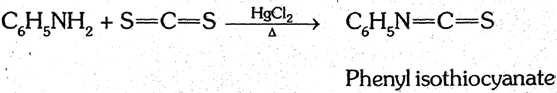
This is a test of aniline and other primary amines.
(6) Reaction with aldehydes : Aniline condenses with aldehydes to form schiff's base.
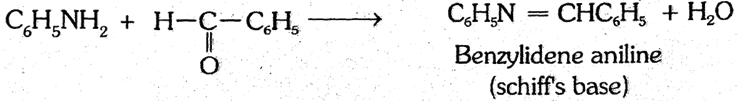
(7) Reaction with hinsberg's reagent :
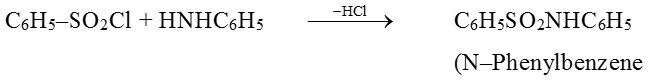
(8) Diazotisation : Diazotisation is a reaction in which ice cooled solution of aniline in an inorganic acid reacts with sodium solution leading to the formation of diazonium salt.
![]()
Benzene diazonium chloride is a useful synthetic reagent. It is used in the preparation of many organic compounds.

(1) Halogenation : In Polar and nonpolar medium Chlorine and bromine react with aniline and form trichloro and tribromo aniline respectively.
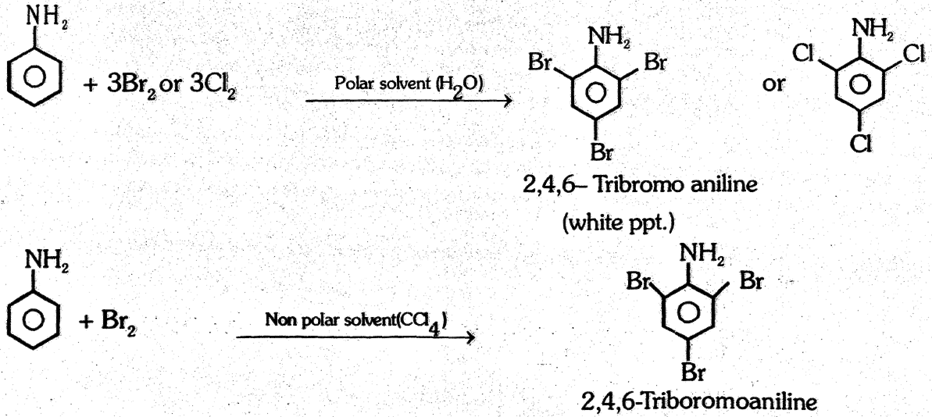
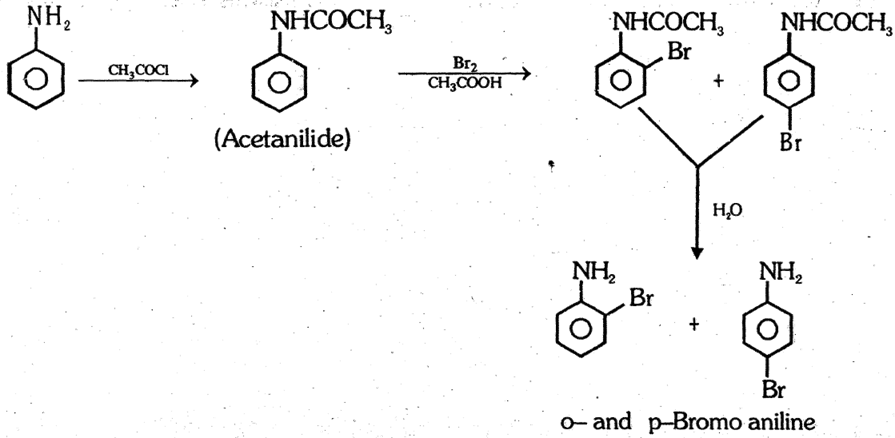
Note : However, monobromo or chloro derivative of aniline can be prepared if –NH2 group is first protected by acetyl group. Here the reactivity decreases due less +M effect on benzene ring.
(2) Nitration :
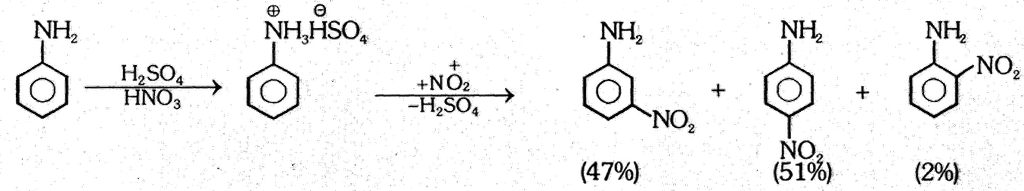
(3) Sulphonation : Aniline reacts with fuming H2SO4 to give sulphanilic acid. (p-Amino-benzene sulphonic acid)
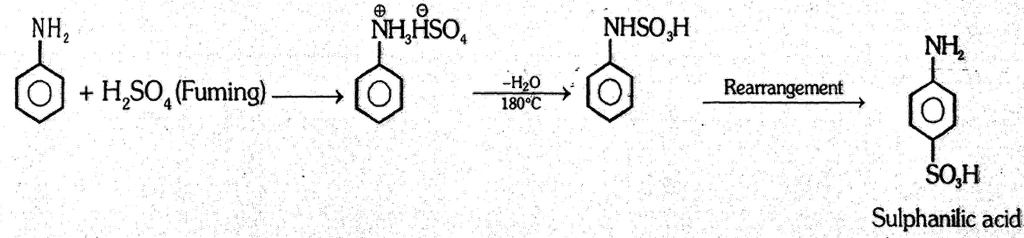
- This process is called baking.
- Sulphanilic acid is an important intermediate in the manufacturing of dyes and durgs.
- The compounds in which both proton donating & proton accepting groups present are called ampholite (dipolare ion)
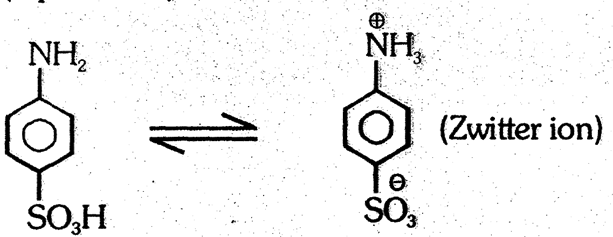
(4) Catalytic hydrogenation: Aniline undergoes hydrogenation in presence of Ni at high temp. to form cyclohexanamine.

Tests of aniline
(i) Carbylamine test : Aniline gives carbylamine test or isocyanide test.
C6H5NH2 + CHCl3 + KOH ¾® C6H5NC (Bad smelling)
(ii) Dye test : Aniline is first diazotised. On adding alkaline solution. Of b-naphthol to the diaztised product a red-orange dye is formed.
(iii) On heating with bromine water, a white ppt. is formed.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
