- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Metal Carbonyls
Compounds of metals with CO as a ligand are called metal carbonyls.
They are of two types.
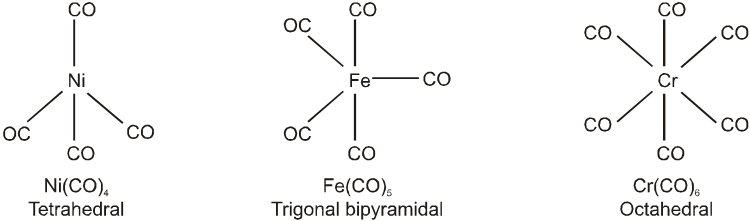
(a) Monomeric : Those metal carbonyls which contain only one metal atom per molecule are called monomeric carbonyls. For examples :[Ni(CO)4] (sp3, tetrahedral) ; [Fe(CO)5 ] (dsp3, trigonal bipyramidal)
[Cr(CO)6] (d2 sp3, octahedral) ; [V(CO)6] (d2 sp3, octahedral, only carbonyl which is paramagnetic having one unpaired electron. This is least stable among all the four carbonyls)
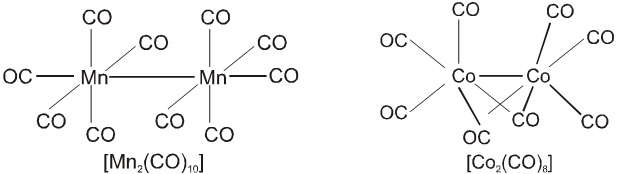
(b) Polymeric : Those metal carbonyl which contain two or more than two metal atom per molecule and they have metal-metal bonds are called polymeric carbonyl. For example : Mn2 (CO)10 , Co2(CO)8, etc.
The metal–carbon bond in metal carbonyls possess both s and p character. The M—C s bond is formed by the donation of lone pair of electrons on the carbonyl carbon (CO is a weak base) into a vacant orbital of the metal. The M — C p bond is formed by the donation of a pair of electron from a filled d orbital of metal into the vacant antibonding p* orbital of carbon monoxide. Thus carbon monoxide acts as s donor (OC ¬ M) and a p acceptor (OC ¬ M), with the two interactions creating a synergic effect which strengthens the bond between CO and the metal as shown in figure.
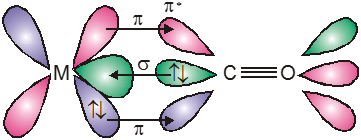
Synergic bonding

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
