1. Alkyl Halides
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Chapter 11: Reaction Mechanism
Introduction :
Alkyl halides :
There are three major classes of organohalogen compounds ; the alkyl halides, the vinyl halides, and the aryl halides.
An alkyl halide simply has a halogen atoms bonded to one of the sp3 hybrid carbon atoms of an alkyl group. (A vinyl halide or Aryl halide has a halogen atom bonded to one of the sp2 hybrid carbon atoms of an aromatic ring. They are different from alkyl halides because their bonding and hybridization are different.)
Classification of halides :
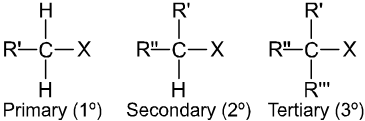
(a) Alkyl halides or haloalkanes (R—X) Compounds Containing sp3 C–X Bond :
They are classified as primary, secondary or tertiary according to the nature of carbon to which halogen is attached.
(b) Allylic halides :
These are the compounds in which the halogen atom is bonded to an sp3–hybridised carbon atom next to carbon-carbon double bond (C=C) i.e. to an allylic carbon.
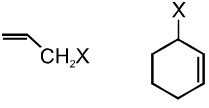
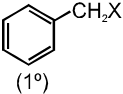
(c) Benzylic halides :
These are the compounds in which the halogen atom is bonded to an sp3–hybridised carbon atom next to an aromatic ring.
(d) Compounds Containing sp2 C–X Bond : Vinylic halides ![]() Aryl halides
Aryl halides ![]()
Structure of alkyl halide :
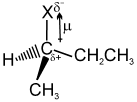
The carbon-halogen bond in an alkyl halide is polar because halogen atoms are more electronegative than carbon atoms. Most reactions of alkyl halides result from breaking this polarized bond. The carbon atom has a partial positive charge, making it some what electrophilic.
2. Types of Reagents
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Types of Reagents :
Reagents are of two types :
(i) Electrophiles
(ii) Nucleophiles
Electrophiles :
Electrophiles are electron deficient species.
Ex. ![]() (positively charged species),
(positively charged species),
![]() (species with vacant orbital at central atom).
(species with vacant orbital at central atom).
Nucleophiles and their nucleophilicity :
Nucleophile is a species having negative charge or lone pair of electrons.
They are electron rich species.
Ex. ![]() (l.p on O-atom),
(l.p on O-atom), ![]() (negaively charged species)
(negaively charged species)
Note : :CCl2 is not a nucleophile because it is electron deficient species and act as electrophile.
- Negative ions have more nucleophilic than their neutral species
![]()
- Down the group nucleophilicity increases because the more polarizable donar atom is better nucleophyle
Polarizability size of donar atom
![]()
- Across the period nucleophilicity decreases
![]()
- Bulky base has less nucleophilic character.
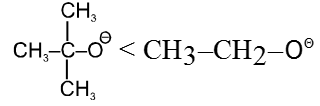
- Effect of solvent : In case of polar aprotic solvents nucleophilicity order of halides is just reversed.
Bases and their basicity :
Bases are the species which accept the proton or which donates l.p. of electron to proton.
- Basicity decreases down the group while nucleophilicity increases
F– > Cl– > Br– > I–
- Nucleophilicity and basicity order will be same across the period.
- For the same donor atom nucleophilicity and basicity order will be same
Leaving group ability :
- Weaker base is better leaving group.
- More resonance stabilised ion will be better leaving group.
- Weaker the carbon-leaving group bond (C–X) better will be the leaving group.
- If activation energy of a reaction is low then reaction will be fast and leaving group will be better.
Ex. (a) I– > Br– > Cl– > F–
(b) CF3SO3– > RCOO– > C6H5O– > OH– >
(c) 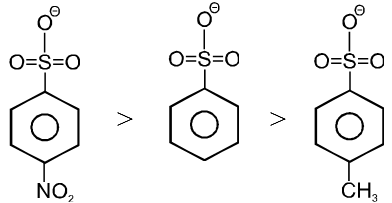
Note : More stable anions are weak bases & hence better leaving group.
3. Types of Solvents
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Types of solvents
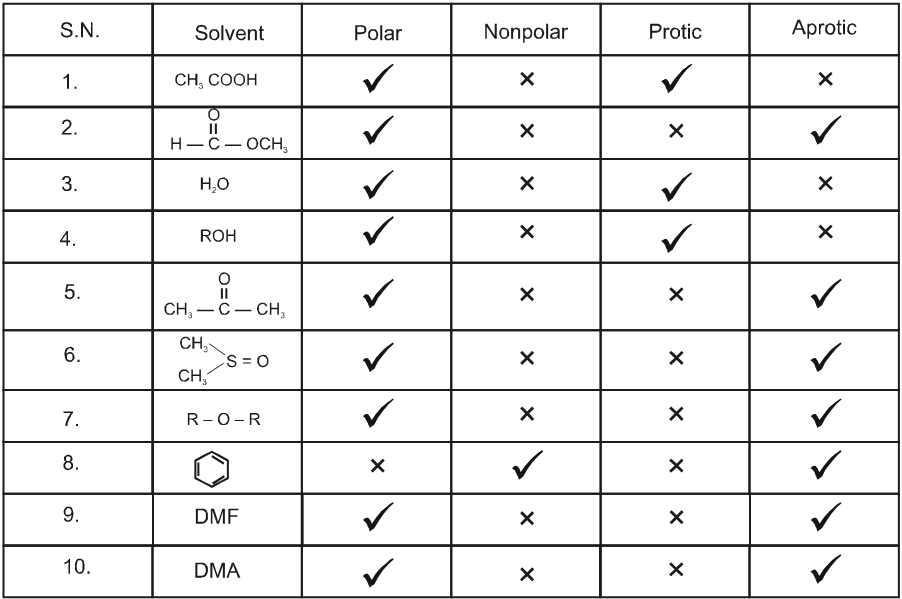
Note : If H atom is attached to oxgyen, nitrogen or sulphur then it is said to be protic solvent.
4. Nucleophilic substitution reactions of alkyl halides
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Nucleophilic substitution reactions of alkyl halides
Alkyl halide undergoes nucleophilic substitution reaction.
![]()
Nucleophilic substitution reactions are of two types :
Unimolecular nucleophilic substitution reaction (SN1) :
- It is a two step process.
- First step is the heterolytic cleavage of carbon halogen bond (
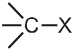 ) to give carbocation intermediate.
) to give carbocation intermediate.
![]() (Ist step is r.d.s.)
(Ist step is r.d.s.)
- In the second step nucleophile attacks from either side of carbocation to generate product (racemic mixture).
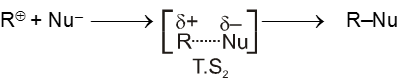 (IInd is fast step)
(IInd is fast step)
Mechanism :
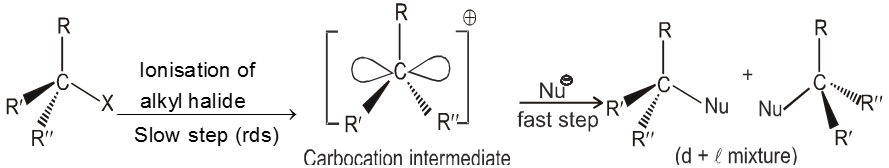
- Carbocation intermediate is formed so rearrangement is possible in SN1 reaction.
Kinetics :
- Rateµ [Alkyl halide]
- It is unimolecular and first order reaction.
- Rate of SN1 reaction is independent of the concentration and reactivity of nucleophile.
Stereochemistry :
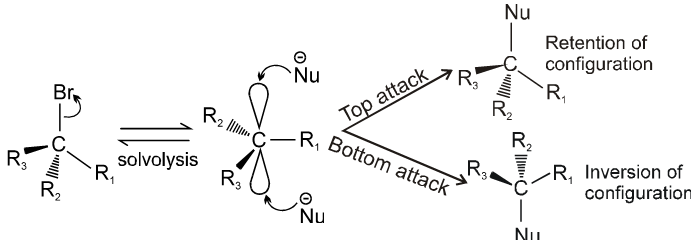
- Catalyst used is Ag+.
- More polar protic solvent is more favourable for SN1.
H2O > ROH > NH3 (order of polar protic solvent).
- In SN1 reaction carbocation is formed along with anion and to solvate these ions, polar protic solvent is used.
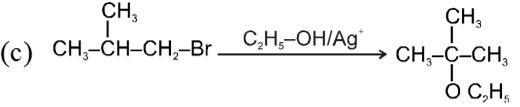
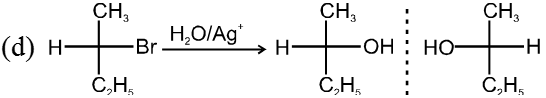
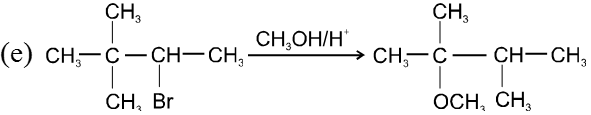
- Decreasing order of reactivity of alkyl halides. ;
R–I > R–Br > R–Cl > R–F
Ex.
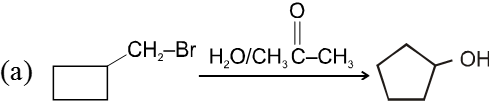
![]()
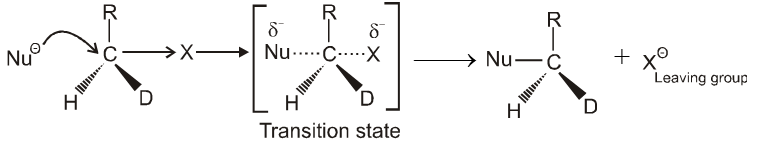
Bimolecular nucleophilic substitution reaction (SN2) :
It is a single step reaction as the rate of reaction depends upon concentration of substrate as well as nucleophile.
Mechanism :
- In this reaction nucleophile attack from back side on the carbon atom bearing leaving group. it is a concerted reaction where bond breaking and bond formation takes place simultaneously to achieve a transition state (trigonal bipyramidal shape) where half bond has been formed and half bond has been broken.
Kinetics :
- rateµ [alkyl halide] [nucleophile]
- It is a bimolecular, one step concerted process.
- It is a second order reaction because in the r.d.s. both species are involved.
Steriochemistry :
Only one product is formed where inversion of configuration takes place.
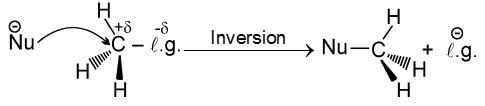
- Polar aprotic solvent is favourable not polar protic solvent. Becuase in case of polar protic solvent nucleophilicity of anion is decreased due to solvation and such solvation is not possible in case of polar aprotic solvent.
- Electron withdrawing group increases the rate of sN2 reaction.
O=CH–CH2–Br > CH3–O–CH2–Br > H–CH2–Br > CH3–CH2–Br
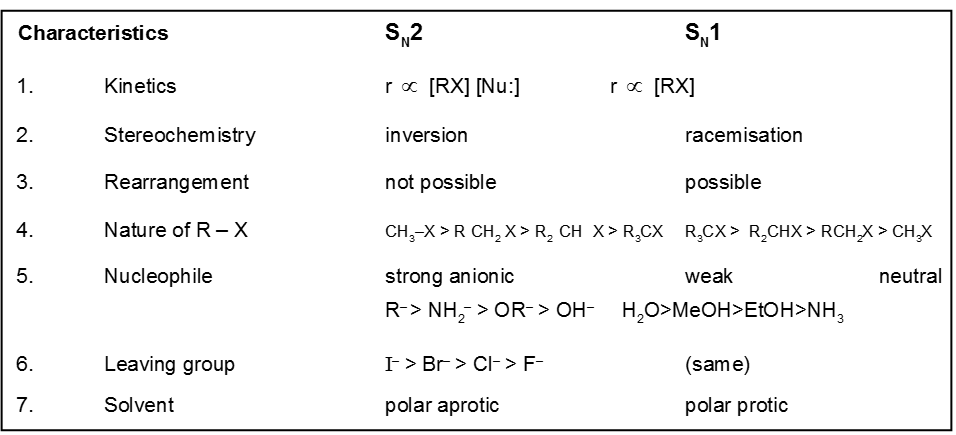
Comparision between SN1 / SN2 reaction :
Examples of SN2 reaction of alkyl halides :
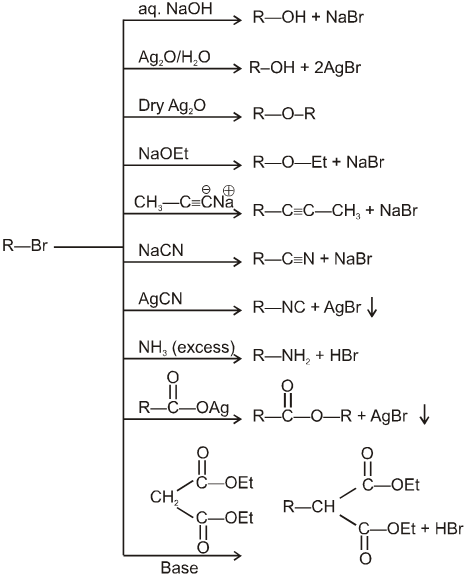
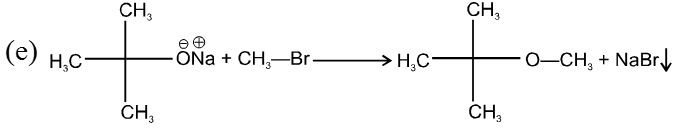
(i) Williamson’s synthesis of ethers :
- It is used for the preparation of symmetrical as well as unsymmetrical ether.
R—Br + NaOEt ¾¾®R—OEt + NaBr
- Williamson’s synthesis involve attack of an alkoxide on alkyl halide to give ethers. In place of alkoxide, phenoxide can also be used.
- This is an SN2 reaction because alkoxide is a strong nucleophile.
- On using 2º & 3º alkylhalide we get alkene not ether as a product.
Ex.
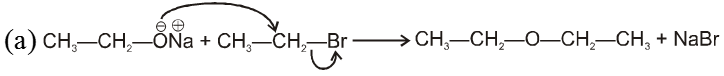
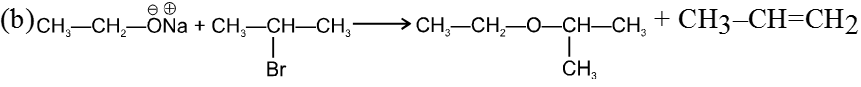
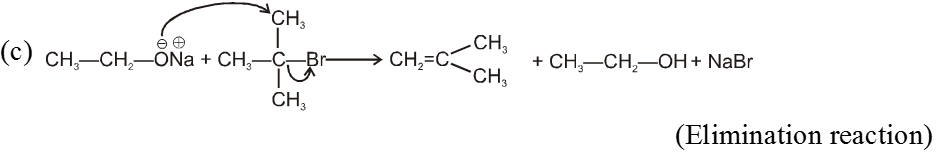
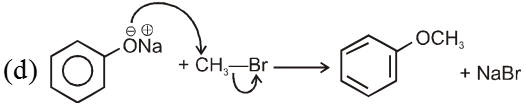
Note : Vinyl or Aryl halide should not be used because they don’t give SN2 reaction.
Ex. 
(ii) Reaction with AgCN and NaCN :
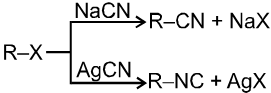
- NaCN is a more ionic hence ionized to give free
 (an ambident nucleophile) where carbon side is more active than nitrogen side. That is why with NaCN, RCN is formed.
(an ambident nucleophile) where carbon side is more active than nitrogen side. That is why with NaCN, RCN is formed.
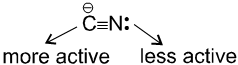
- On the other hand AgCN is more covalent so it is not ionized therefore only nitrogen side is free to act as a nucleophile and give isocyanide (R—NC)
(iii) Reaction with NH3 :

![]()
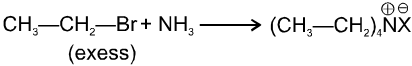
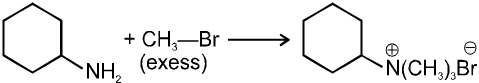
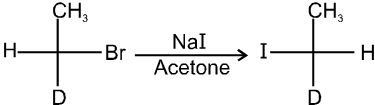
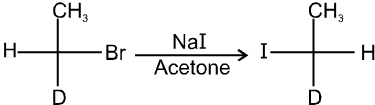
(iv) Finkelstein reaction :
R—OH + HI¾¾®R—I ![]() R—H + I2 (reduced) [Final product is Alkane (R-H) not R-I]
R—H + I2 (reduced) [Final product is Alkane (R-H) not R-I]
This is a problems that is why iodides are best prepared through halogen exchange reaction. It is also known as Finkelstein reaction. In this reaction R—Cl and R—Br is treated with sodium iodide in acetone.
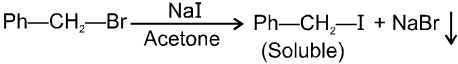
![]()
- NaI is soluble in acetone. In this reaction acetone is used because sodium iodide is soluble in acetone but NaBr and NaCl are insoluble so precipitated out. This eliminates any possibility of reverse reaction.
- It is an SN2 reaction therefore only 1ºRX and 2ºRX is used.
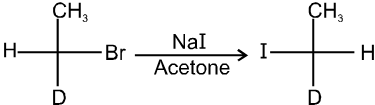
5. Aryl Halides
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Aryl Halides
Preparation of Aryl Halides
1. By Halogenation :
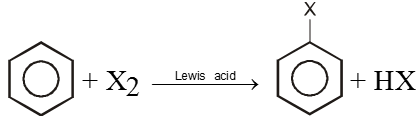
X2 = Cl2, Br2 ; Lewis acid = FeCl3, AlCl3, ZnCl2, etc.
2. By Decarboxylation :
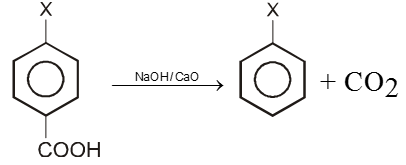
Chemical reaction of Aryl halide (Bimolecular nucleophilic substitution SN2 Ar)
- An electron withdrawing group at ortho or para positions with respect to a good leaving groups are necessary conditions for SN2 Ar.
Mechanism :
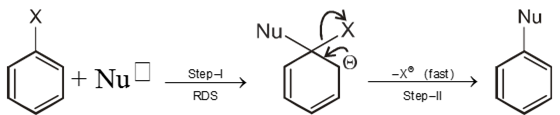
Intermediate ion is stabilized by resonance and are stable salts called Meisenheimer salts.
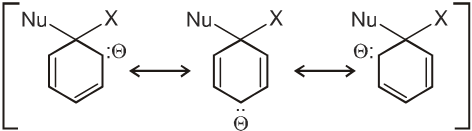
- A group that withdraws electrons tends to neutralize the negative charge of the ring and this dispersal of the charge stabilizes the carbanion.
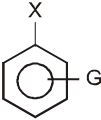
G withdraws electrons : stabilizes carbanion, activates the Ar-SN2 reaction.
(–![]() (CH3)3, –NO2, –CN, –SO3H, –COOH, –CHO, –COR, –X)
(CH3)3, –NO2, –CN, –SO3H, –COOH, –CHO, –COR, –X)
- A group that releases electrons tends to intensify the negative charge, destabilizes the carbanion, and thus slows down reaction.
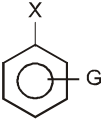
G (–NH2, –OH, –OR, –R) releases electrons : destabilizes carbanion, deactivates the Ar-SN2 reaction.
Element effect :- The fact that fluoro is the best leaving group among the halogens in most ArSN2 but in SN1 & SN2 mechanism where fluoro is the poorest leaving group among halogens.
6. Elimination reactions of alkyl halides
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Elimination reactions of alkyl halides
In an elimination reaction two atoms or groups (YZ) are removed from the substrate and generally resulting into formation of p bond.
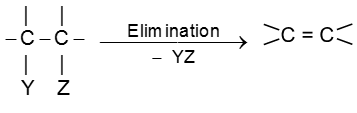
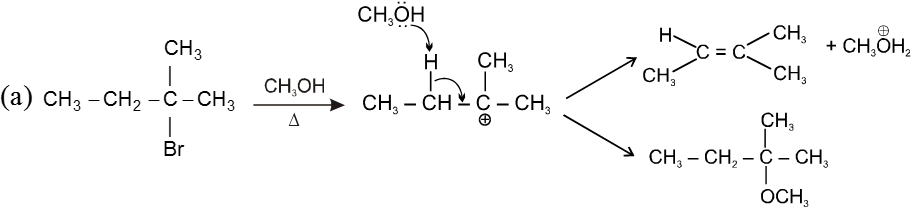
Unimolecular elimination reaction (E1) :
Proton and leaving group depart in two different step.
Mechanism :
Step 1 : Formation of the carbocation (r.d.s.)

Step 2 : Base ( ) abstracts a proton (fast step)

- Reaction intermediate is carbocation, so rearrangment is possible
Kinetics:
- Rateµ[Alkylhalide]
- It is a unimolecular and first order reaction.
- Reactivity order is similar to SN1 becuase Carbocation Intermediate is formed in the rds step.
SN1 v/s E1 :
- In case of alkyl halides SN1 product is generally more than E1 product
Ex.
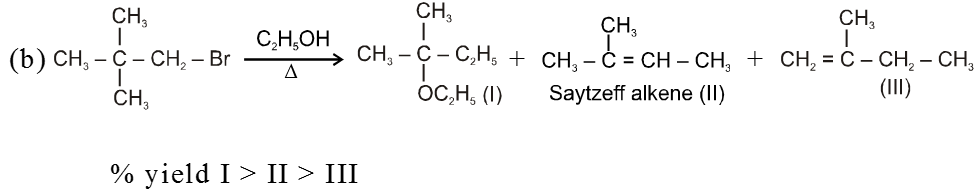
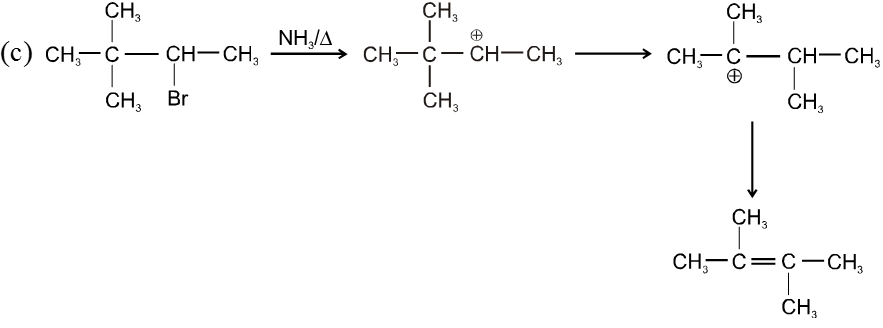
Bimolecular elimination reaction (E2) :
- It is a second order reaction because rate of reaction depends upon conc. of substrate as well as base.
Mechanism :
- Orientation of eliminated proton and leaving group are antiparallel or antiperiplanar to each other because in anti conformation the transition state is more stable due to minimum electronic repulsion.
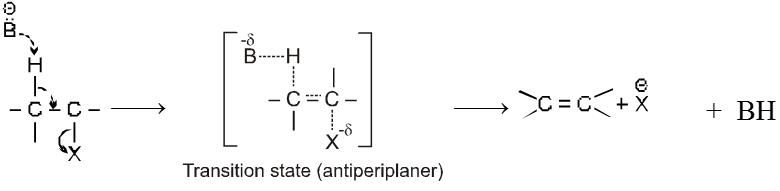
- E2 reaction is stereospecific.
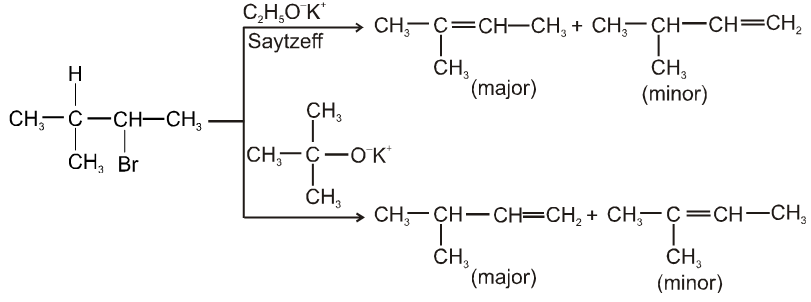
- E2 reaction also depends upon size of base which decides major or minor product.
Kinetics :
- Rate µ [R – X] [Base]
- This is a single step, bimolecular reaction
- No carbocation intermediate is formed hence there is no rearrangement but a transition state is achieved because it is a single step reaction.
- More favourable substrate is tertiary alkyl halide because it will give more stable alkene according to saytzeff rule.
Ex.
Some important terms :
(a) Regioselective reaction : Those reaction in which more than one structural isomeric products are possible, said to be regioselective reaction.
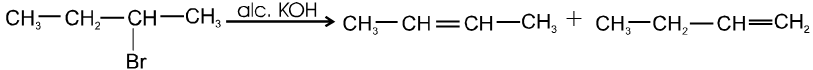
(b) Regiospecific reaction : Those reaction in which only one structural isomer product is formed out of the possible products, said to be regiospecific reaction.
(c) Stereoselective : Those reactions in which mixture of two stereoisomeric products are formed with one major product.
Examples : SN1, SN2 and E2
(d) Stereospecific reaction : Those reactions in which two stereoisomeric reactants give two different stereoisomeric products, are called as stereospecific reactions.
Examples : SN2 and E2
Note : All stereospecific reactions are stereo selective.
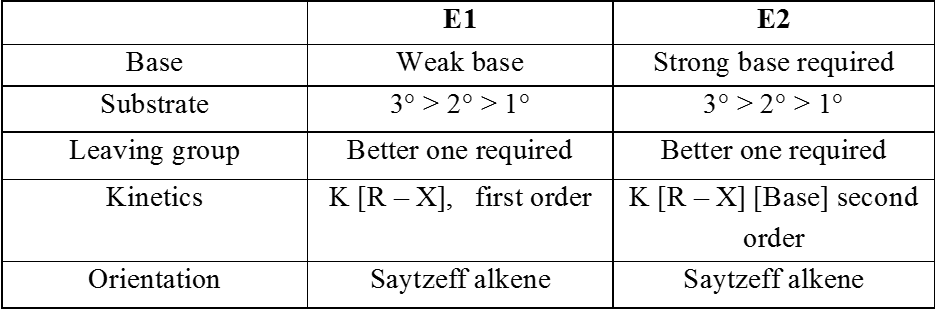
Comparison between E1 and E2 reactions :
E1cB Reaction :
- It is two step reaction.
- In first step base takes proton from adjacent carbon atom of halogen bearing carbon and generate carbanion intermediate.
-
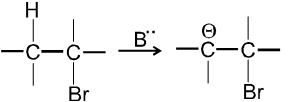
- In second step there is loss of leaving group by carbanion to get alkene :
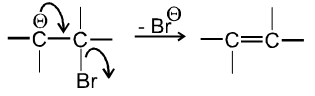
Ex.
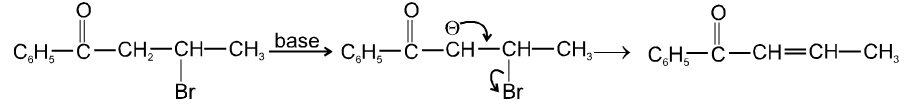
- b-hydrogen atom should be more acidic which is possible only if carbon atom having b-hydrogen atom should be link to with electron withdrawing group (-m, -I group).
- Leaving group should be more electronegative because it also increases acidity of b-hydrogen atom.
- Experimentally it is found that Ist step i.e. formation of carbanion intermediate is fast step and second step i.e. removal of leaving group is slow step thus r.d.s. in E1cB is second step.
7. Reactions of chloroform
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Reactions of chloroform
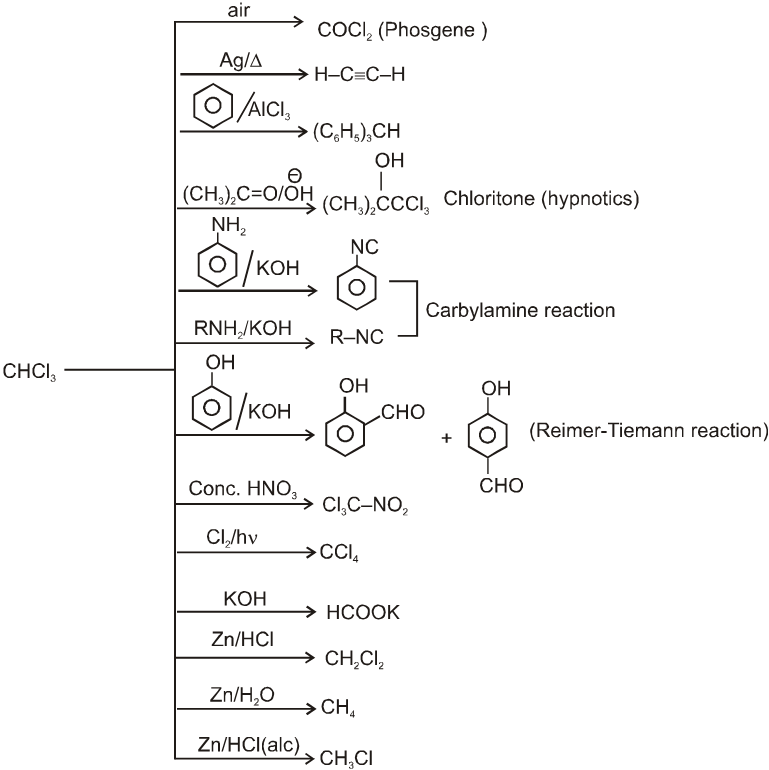
- Purity of chloroform (presence of phosgene) can be tested before use as anaesthetic by treating with aqueous solution of AgNO3 because the presence of COCl2 may cause cardiac failure.
- Chloroform is stored in dark colour bottle containing small amount of ethyl alcohol. (It converts phosgene into diethylcarbonate).
8. Carbon tetrachloride (pyrene)
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Carbon tetrachloride (pyrene)
Preparations & reactions :
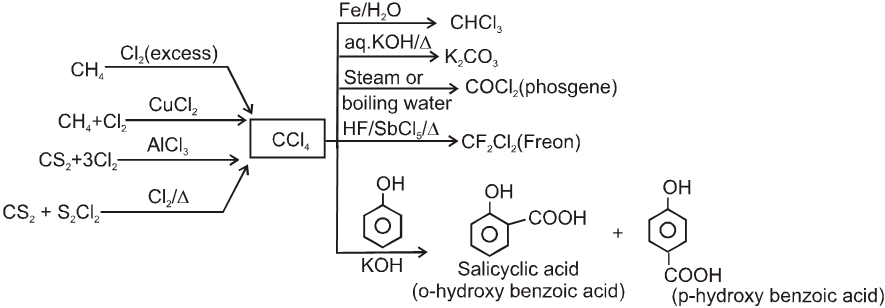
- CCl4 is stable to heat and its vapours do not catch fire thus it is used as fire extinguisher.
- CCl4 used as medicine for elimination of hook worms.
Freons (Polychlorofluoro alkane)
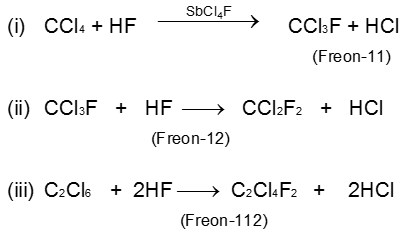
Nomenclature of Freons :
Freon is expressed as
Freon - cba
Where
a – [Number of F-atoms]
b – [1 + Number of H–atoms]
c – [Number of C-atoms –1]
Example : C2Cl4F2 is expressed as Freon-112
a = 2 ; b = 1 + 0 ; c = 2 – 1
cba = 112
- Freon is odourless, non-corrosive, non-toxic gas. It can easily be liquefied hence widely used as refrigerant.
9. Alchols and Ethers – Introduction and Classification
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Alchols and Ethers – Introduction and Classification
Alcohols :
Alcohols have sp3 hybridized oxygen atoms, but the C – O – H bond angle in methanol (108.9°) is considerably larger than the H – O – H bond angle in water (104.5°) because the methyl group is much larger than a hydrogen atom.
The bulky methyl group counteracts the bond angle compression caused by oxygen’s nonbonding pairs of electrons. The O – H bond lengths are about the same in water and methanol (0.96 Å), but the C – O bond is considerably longer (1.4 Å), reflecting the larger covalent radius of carbon compared to hydrogen.

Classification of alcohols:
(a) Alcohols may be classified as mono–, di–, tri- or polyhydric alcohols depending on whether they contain one, two, three,.............hydroxy group.
Monohydric : Contains one –OH group (C2H5OH ; CH3–CH2–CH2–OH)
Dihydric : Contains two –OH group ![]() (Glycol)
(Glycol)
Trihydric (Polyhydric): Contains three or more than three –OH groups ![]() (Glycerol)
(Glycerol)
(b) Allylic alcohols: CH2=CH–CH2–OH ; 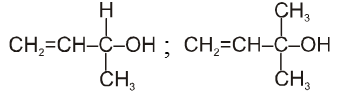 ;
;
(c) Vinylic Alcohol: CH2=CH–OH (unstable at room temperature)
(d) Benzylic alcohols:
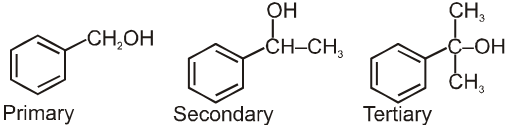
Ethers :
Like water, ethers have a bent structure, with an sp3 hybrid oxygen atom giving a nearly tetrahedral bond angle.
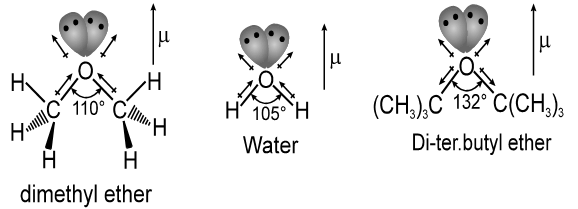
Bonding in ethers is readily understood by comparing ethers with water and alcohol. Van der Waals strain involving alkyl groups causes the bond angle at oxygen to the larger in ethers than in alcohol, and larger in alcohols than in water. An extreme example is di-tert-butyl ether, where steric hindrance between the tert-butyl groups is responsible for a dramatic increase in the C – O – C bond angle.
Classificiation of ethers :
(a) Simple or symmetrical : If the alkyl or aryl groups attached to the oxygen atom are the same.
CH3OCH3, C2H5OC2H5
(b) Mixed or unsymmetrical : If the two groups attached to the oxygen atom are different.
C2H5OCH3, C2H5OC6H5.
General Reactions of alkyl halides, alcohols & ethers :

10. Nucleophilic substitution (SN) reaction of alcohols
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Nucleophilic substitution (SN) reaction of alcohols
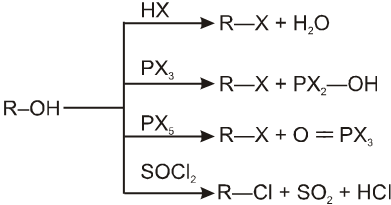
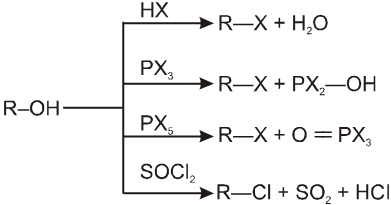
In presence of HX :
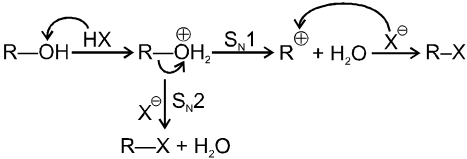
R–OH + HX RX + H2O
This is a nucleophilic substitution (SN1/SN2)
Mechanism :
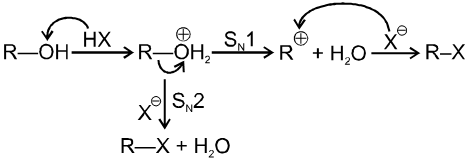
Note : Mainly - unsubstituted 1º alcohol give SN2 reaction with HX.
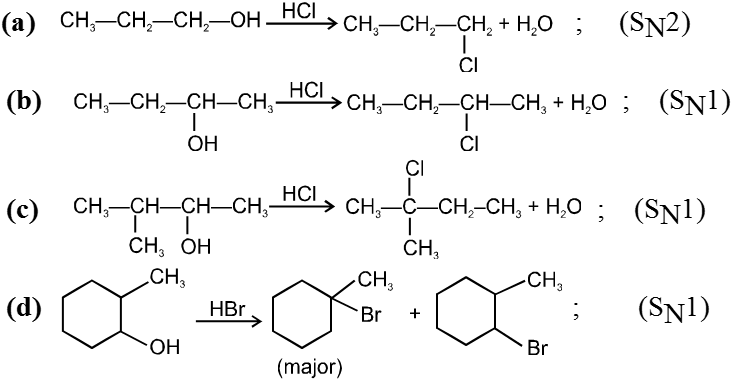
Ex.
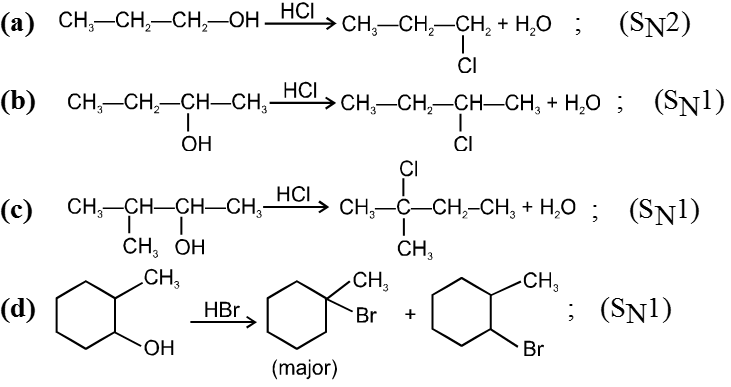
Reactivity of HX is HI > HBr > HCl > HF.
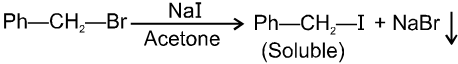
Finkelstein reaction :
R—OH + HI R—I  R—H + I2 (reduced) [Final product is Alkane (R-H) not R-I]
R—H + I2 (reduced) [Final product is Alkane (R-H) not R-I]
This is a problems that is why iodides are best prepared through halogen exchange reaction. It is also known as Finkelstein reaction. In this reaction R—Cl and R—Br is treated with sodium iodide in acetone.
![]()
![]()
NaI is soluble in acetone. In this reaction acetone is used because sodium iodide is soluble in acetone but NaBr and NaCl are insoluble so precipitated out. This eliminates any possibility of reverse reaction.
It is an SN2 reaction therefore only 1ºRX and 2ºRX is used.
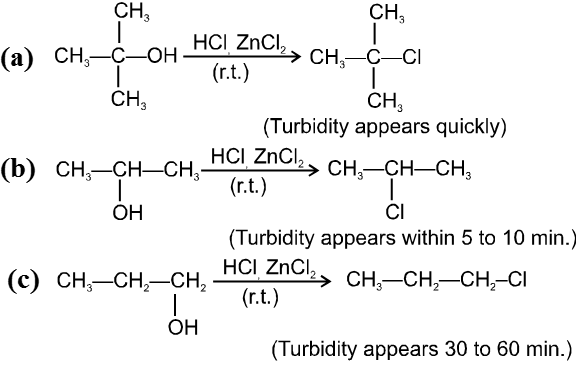
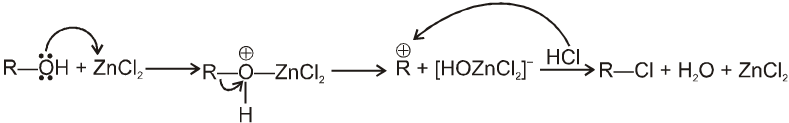
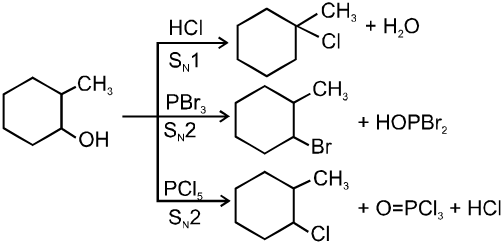
Lucas reagent :
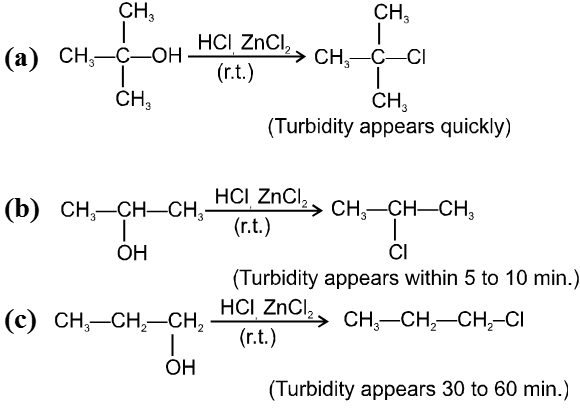
The 1 : 1 mixture of anhydrous ZnCl2 : HCl (conc.) is called Lucas reagent which is used to distinguish between 1º, 2º and 3º alcohols.
![]()
Mechanism :
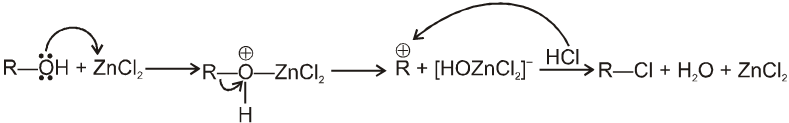
ZnCl2 increases the rate of reaction by making –OH group into a much better leaving group just through complexation.
Reactivity of alcohols is 3ºROH > 2ºROH > 1ºROH
Ex.
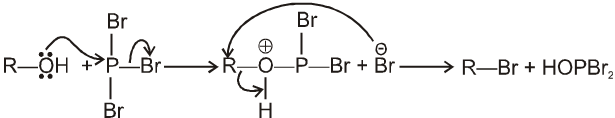
1.4 In presence of PX3 :
R–OH  R–Br
R–Br
Mechanism :

2R—OH + HOPBr2 2R–Br +
1.5 In presence of PX5 :

Both the PX3 & PX5 proceeds via SN2 pathways, No.rearrangement occurs.
Only 1ºR—OH and 2ºR—OH undergo this types of reaction. 3º R—OH undergoes elimination reaction.
Ex. 
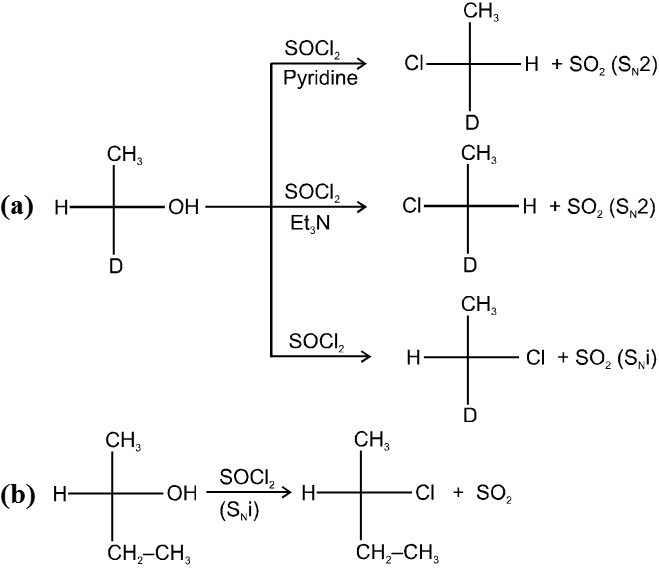
1.6 In presence of SOCl2 (Thionyl chloride) :
R—OH R—Cl + SO2 (SN2) ; R—OH
R—Cl + SO2 (SN2) ; R—OH  R—Cl + SO2 (SNi)
R—Cl + SO2 (SNi)
(A) Mechanism of SOCl2 with pyridine :

SOCl2 with pyridine undergo SN2 reaction so inversion of configuration is obtained in product.
(B) Mechanism of SOCl2 without pyridine : (Darzen method)

SOCl2 without pyridine or base undergo SNi reaction, so retention of configuration is obtained in product.
Ex. (a) 
(b) 
1.7 Victor Mayer test :
1° Alcohol%

2° Alcohol%

3° Alcohol %

11. E1 Reaction of alcohols
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
E1 Reaction of alcohols
For E1 mechanism reagents are H3PO4 /D, H2SO4 / 160º
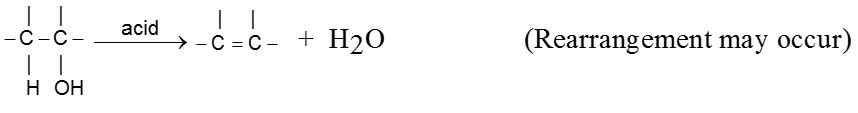
Mechanism :
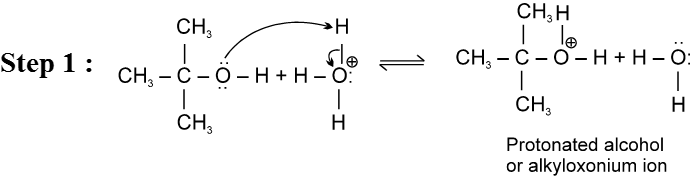
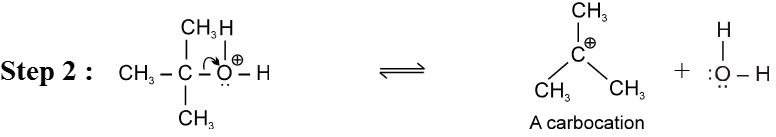
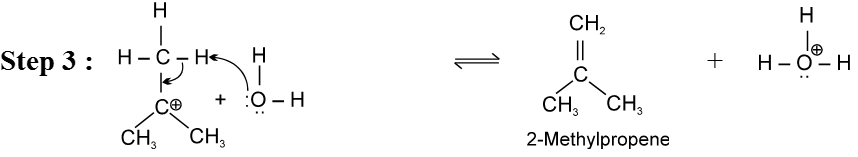
- Reactivity of ROH is 3° > 2° > 1°.
Ex.
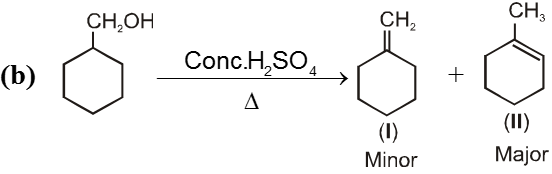
12. E2 Reaction of alcohols
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
E2 Reaction of alcohols
Alcohols gives E2 reaction with Al2O3, P2O5, ThO2, WO3
![]()
- With Al2O3 and P2O5 Saytzeff alkene is major product but with ThO2 and WO3 Hofmann alkene is major product.
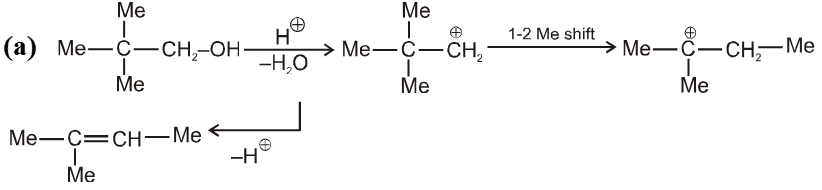
Pinacol-Pinacolone rearrangement :
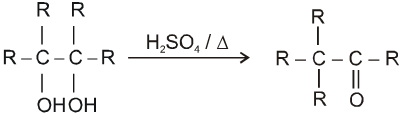
Mechanism :
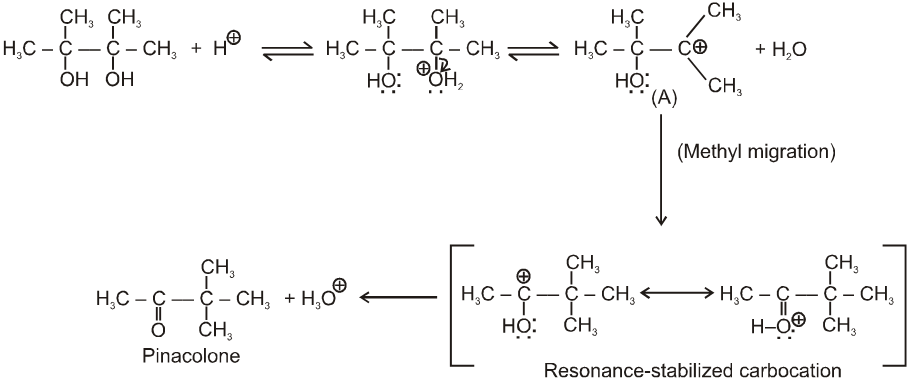
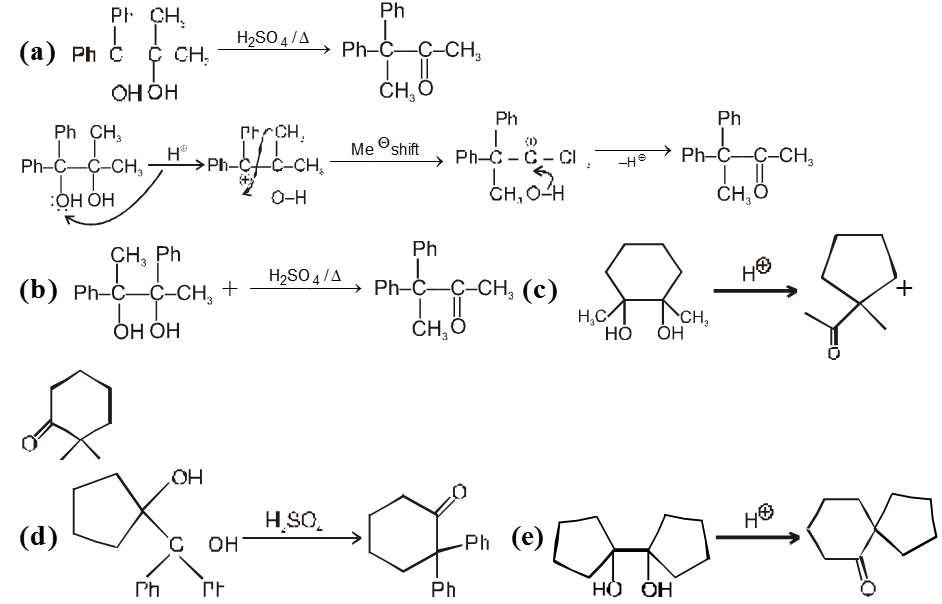
Note : For unsymmetrical diol that –OH group will be replaced first which will generate stable carbocation as below .
Ex.
13. Ethers
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Ethers
Preparation of ether :
(i) From Wiliamsons synthesis method :
![]()
Ex.
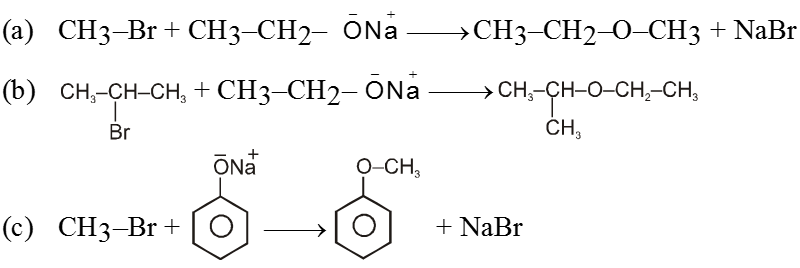
(ii) By using dry Ag2O :
![]()
Ex.
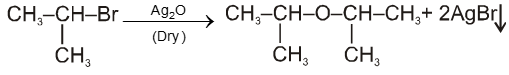
(iii) From alcohols :
![]()
(Trace)
- By intermolecular dehydration of alcohols ethers can be prepared in presence of trace amount of H2SO4.
Mechanism :
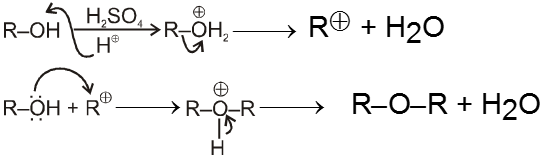
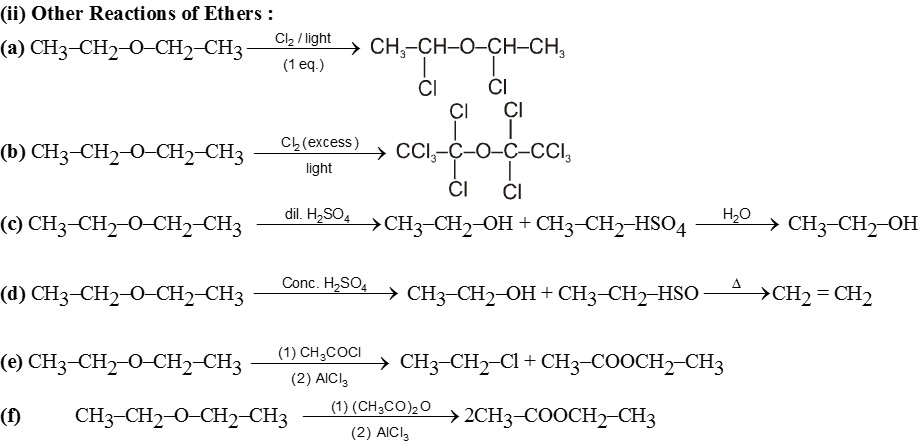
Purification of ethers :
On standing in contact with air they form unstable peroxide (R2O ® O) which is explosive in nature. Peroxide is formed at the carbon atom next to the etherial oxygen.
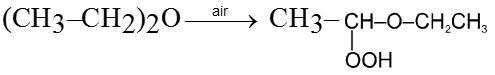
- The presence of peroxide in ether can be identified on shaking it with freshly prepared ferrous sulphate solution followed by addition of potassium thiocyanate. This result into red colour of ferric thiocyanate. During the reaction peroxide oxidises Fe2+ to Fe3+ which reacts with thiocyanate ion to give red colour of ferric thiocyanate.
![]()
(Red colour)
- Ethers are purified from peroxide before use. The peroxide from ether can be removed by treating ether with KI solution where peroxide is reduced to ether and iodide ion is oxidised to I2 molecule.
- Peroxide formation can be checked by adding few ammount of Cu2O in ether
- Ether is lewis base hence forms co-ordinate bond with lewis acid
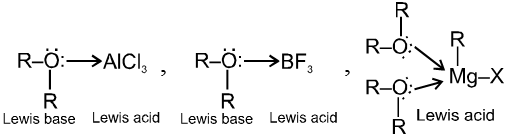
Chemical properties of ethers :
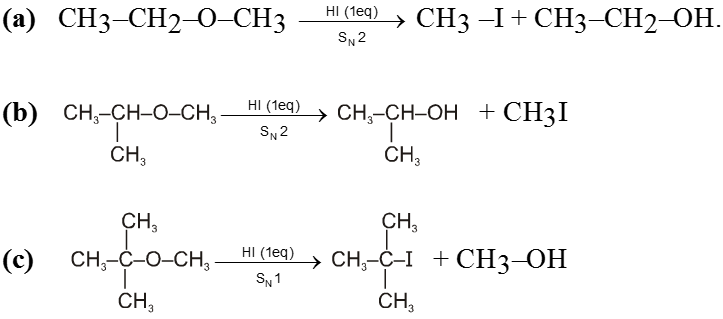
(i) Reaction with HI :
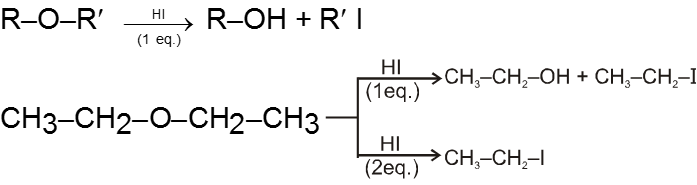
- Those protic acids whose conjugate base is a good nucleophile leads to cleavage of ethers. HI is such a very prominent acid of this kind.
- In most cases of unsymmetrical ethers 1 eq. of HI leads to give a mixture of four products in which it is not possible to predict the major product. However if 2 eq. of HI is used then two iodides are obtained.
Ex.
10. Nucleophilic substitution (SN) reaction of alcohols
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Nucleophilic substitution (SN) reaction of alcohols
In presence of HX :
![]()
This is a nucleophilic substitution (SN1/SN2)
Mechanism :
Note : Mainly b - unsubstituted 1º alcohol give SN2 reaction with HX.
Ex.
- Reactivity of HX is HI > HBr > HCl > HF.
Finkelstein reaction :
![]() (reduced) [Final product is Alkane (R-H) not R-I]
(reduced) [Final product is Alkane (R-H) not R-I]
This is a problems that is why iodides are best prepared through halogen exchange reaction. It is also known as Finkelstein reaction. In this reaction R—Cl and R—Br is treated with sodium iodide in acetone.
![]()
- NaI is soluble in acetone. In this reaction acetone is used because sodium iodide is soluble in acetone but NaBr and NaCl are insoluble so precipitated out. This eliminates any possibility of reverse reaction.
- It is an SN2 reaction therefore only 1ºRX and 2ºRX is used.
Lucas reagent :
The 1 : 1 mixture of anhydrous ZnCl2 : HCl (conc.) is called Lucas reagent which is used to distinguish between 1º, 2º and 3º alcohols.
![]()
Mechanism :
ZnCl2 increases the rate of reaction by making –OH group into a much better leaving group just through complexation.
Reactivity of alcohols is 3ºROH > 2ºROH > 1ºROH
Ex
In presence of PX3 :
![]()
Mechanism :
2R—OH + HOPBr2¾¾® 2R–Br +
In presence of PX5 :
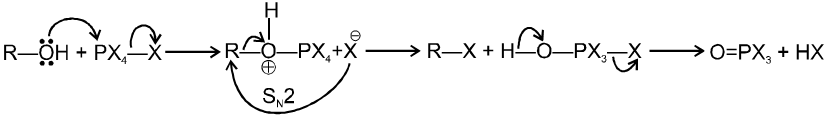
Both the PX3 & PX5 proceeds via SN2 pathways, No.rearrangement occurs.
Only 1ºR—OH and 2ºR—OH undergo this types of reaction. 3º R—OH undergoes elimination reaction.
Ex.
In presence of SOCl2 (Thionyl chloride) :
![]()
(A) Mechanism of SOCl2 with pyridine :
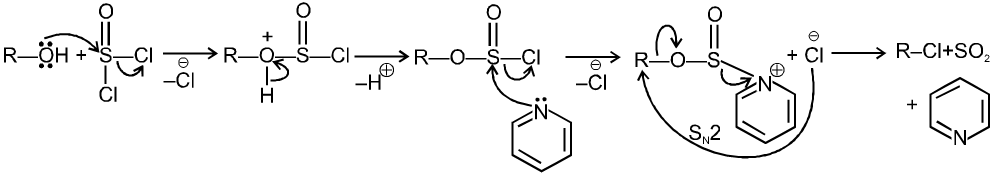
SOCl2 with pyridine undergo SN2 reaction so inversion of configuration is obtained in product.
(B) Mechanism of SOCl2 without pyridine : (Darzen method)
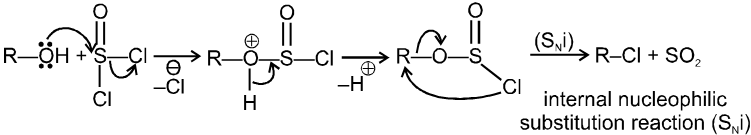
SOCl2 without pyridine or base undergo SNi reaction, so retention of configuration is obtained in product.
Ex.
Victor Mayer test :
1° Alcohol%
![]()
2° Alcohol%
![]()
3° Alcohol %
![]()
Cerric Ammonium Nitrate test :
Alcohols(1°,2°,3°) also give characteristic red colour with cerric ammonium nitrate [(NH4)Ce(NO3)6] solution.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
