1. Drugs and their Classification
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Chapter 15: Chemistry in Everyday Life
1. Introduction to Drugs and Chemotherapy :
In general the drug may be defined as the substances used in the prevention, diagnosis, treatment or cure of disease in man or animals.
When administered to the ailing individual, its action should be localised at the site where it is desired to act. (In actual practice, there is no drug which behaves in this manner).
Chemotherapy :
“The use of chemicals to destroy infectious micro organisms without causing any injury to the host is called as chemotherapy” .
Drugs and Classification of drugs
(a) On the basis of Pharmacological effect
(b) On the basis of drug action
(c) On the basis of chemical structure
(d) On the basis of molecular targets
Drug Target Interaction :
Macromolecules of biological origin perform various functions in the body for example proteins which perform the role of biological catalysts in the body are called enzymes, and those which are crucial to communication system in the body are called receptors.
Enzymes as Drug Targets :
(a) Catalystic action of enzymes :
In catalytic activity, enzymes perform two major functions as follows
(i) To hold the substrate for chemical reaction :
(ii) The second function of the enzyme is to provide functional group which will attacks the substrate to carry out chemical reaction.
Receptors as Drug Targets :
Receptors are proteins that are crucial to body’s communication process.
2. Types of Chemical Messenger
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Types of chemical messengers
There are two types of chemical messengers :
- Hormones
- Neurotransmitters.
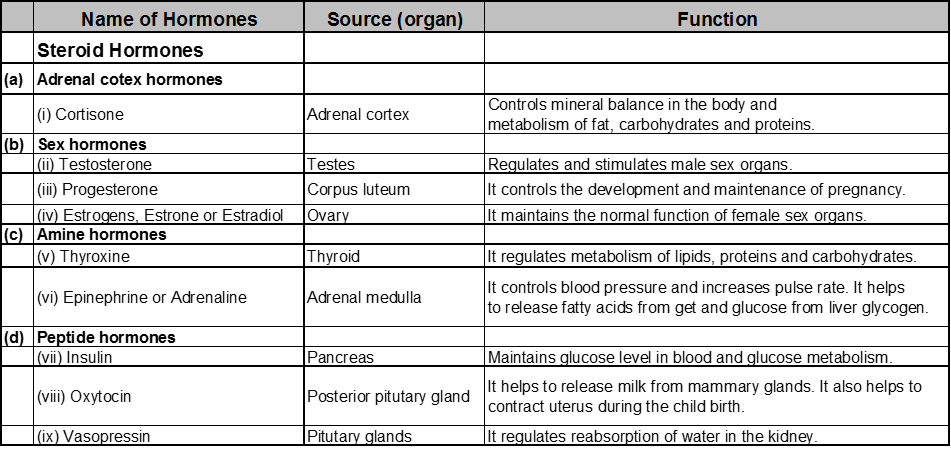
(i) Hormones :
Classification of hormones : Based on their chemical structure ; hormones fall into three classes.
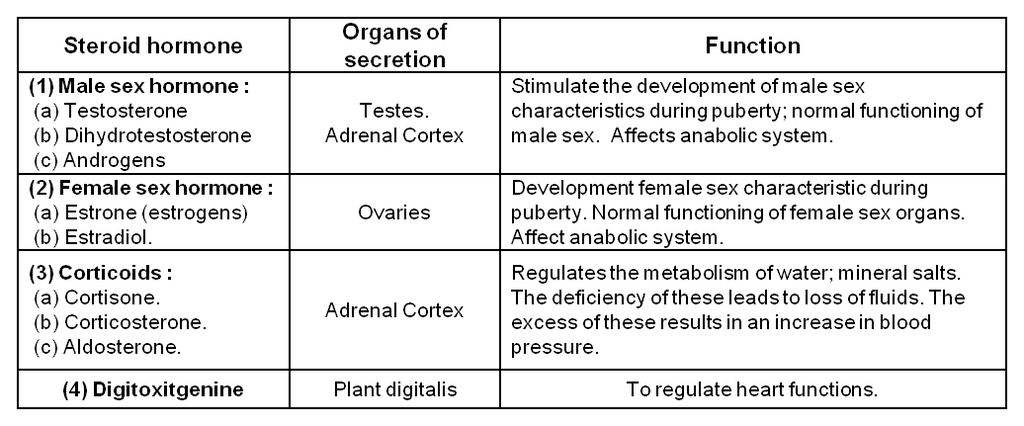
Steroid hormones :
Peptide hormones :

Amine hormones :
Those which are neither steroids nor peptides. These are water soluble amine compounds.
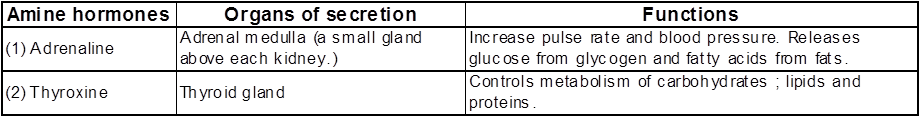
(ii) Neurotransmitters :
*Nerves transfer message through neurotransmitters.
*There are small molecules such as acetyl choline, dopamine and serotonin.
3. Drugs According to their Action
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Drugs According to their Action :
(1) Antacids :
*The chemicals which are used to reduce the acidity of the stomach are called antacids.
*Antacids are nature is basic. Their PH value is in the range of 7.0 to 8.0.
eg. : Omeprazole, Lansoprazole
(2) Antihistamines or Antiallergic Drugs :
*Antihistamines are the drugs which diminish or abolish the effects of histamine.
eg. : Histamine, Cimetidine, Ranitidine
Allergy :
Allergy may be defined as the hypersensitive reponse of the body of certain persons to the external stimulus (such as some drugs, foods, dust, pollen grains, catfur, fabrics etc.)
*The substances which cause allergy are called allergens.
*Most commonly used anti-histamine under the trade name avil :
(3) neurologically Active Drugs
(a) Tranquilizers :
*The chemicals which are used to reduce mental tension, relieve anxiety and mental stress are called Tranquilizer. They act on central nervous system and are hypnotics.
*Tranquilizers are effective in such mental disorder when ordinary hypnotics or sedatives fail. These are called as psychotherapeutic drugs.
eg. : Barbituric acid, Luminal, Serotonin, Bithional
Note : * Reserpine, an alkanoid, is a powerful tranquillzer. It is obtained from a plant, Rauwolfia serpentina (common name - Sarpagandha) which grows in india.
(b) Analgesics : The chemicals which are used for relieving pain are called ANALGESICS.
(i) Non-narcotic analgesics (Non addictive) : Aspirin and Paracetamol belong to this class.
*These drugs also act as antipyretic (reducing fever), and preventing platelet coagulation.
(ii) Narcotic Analgesics :
*Morphine and its homolgues in medicinal doses, relieve pain and produce sleep. In higher doses these produce STUPOR , COMA, CONVULSIONS and ultimately death.
*The narcotics are mainly used for the relief of postoperative pain, cardiac pain and pain of terminal cancer, and in child birth.
eg. : 2-Acetoxy benzoic acid (Aspirin), Paracetamol (4-acidamido phenol), Ibuprofen
(4) Antimicrobials :
*The chemicals which stop the growth or kill the micro organism such as bacteria, virus, fungi, moulds etc are called antimicrobials.
* Antibiotics, antiseptics and disinfectants are antimicrobial drugs.
(a) Antibiotics : *The chemicals produced by micro organisms like bacteria, fungi and moulds that inhibit the growth or destory other micro organism causing infectious diseases in men or animal’s body are called antibiotics. *Alexander fleming in 1929 first.
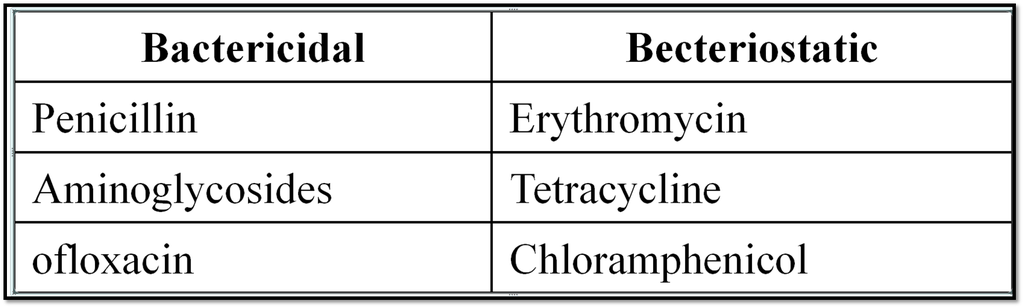
(I) Penicillin : *Six types of penicillines have been isolated so far. Among them penicillin-G is most widely used and is narrow spectrum.
*Ampicillin and amoxicilline are synthetic modification of penicilline and these has broad spectrum effect.
Penicillin is used for the treatment of pneumonia bronchitis bounds etc.
(II) Streptomycin : It is an effective broad spectrum antibioctic. It is used for the treatment of tuberculosis, meningitis and pneumonia
(III) Tetracyclins : Teramycin and oriomycin are important examples of this class of antibiotics. Teramycin is used for the treatment of typhoid and oriomycin is used for the the treatment of eyes.
(IV) Chloramphenicol : It is marketed as chloromycetin and is used for the treatement of typhoid, dysentery pneumonia, meningitis etc.
(b) Antiseptic and Disinfectants :
*Antiseptics are applied to the living tissues such as wounds, cuts ulcers and diseased skin surface. Examples are furacin, soframycin etc.
*Commonly used antiseptic is detol, it is a mixture of chloroxylenol and terpineol.
*Bithional is added to soaps to impart antiseptic properties.
*Iodoform is also used as antiseptic for wounds, boric acid in dilute aqueous solution is weak antiseptic for eyes.
Disinfactants are the substances which applied to inanimate objects such as floors drainage system instruments etc.
*One substance can act as an antiseptic and also act as disinfactant for example
(a) 0.2 percent solution of phenol is an antiseptic while its 1% solution is disinfectant.
(b) Chlorine in 0.2 to 0.4 ppm in aqueous solution is used to disinfect drinking water.
(c) Hexachlorophen :- It is mainly used in soaps creams and emulsions.
(d) Thymol : It is a natural derivative of phenol and is a powerfull disinfectant
(e) Amyl meta cresol (5-methyl-2-pentyl phenol) it is used as antiseptic in mouth wash or gargles.
(f) Gention violet and methylene blue are organic dyes but used as effective antiseptic.
(c) Sulpha Drugs :
A group of drugs which are derivatives of sulphanilamide are known as sulpha drugs.
eg. : Sulphadiazine, Sulphapyridine
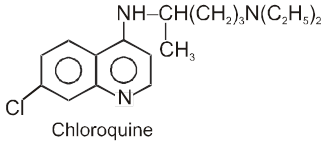
(d) Antimalarials :
*These are the medicines of malaria. In earlier days malaria was treated with the bark of cinchona tree.
* The chloroquine phosphates are sold in the market under the trade name - resochin, ciplaquine, nivaquine etc.
(e) Antifungal Drugs :
*These are drugs used for superfical and deep (systemic) fungal infections.
*Two important antibiotics used as antifungal drugs, introduced way back in 1960, are amphotericin-B and griseofulvin.
(f) Antiamoebic Drugs :
*These are drugs useful in infection, caused by the protozoa Entamoeba histolytica.
*Metronidazole, tinidazole and tetracyclines are important antiamoebic drugs, used these days.
(g) Antiviral Drugs :
* Viruses are the ultimate expression of parasitism; they not only take nutrition from the host cell but also direct its metabolic machinery to synthesize new virus particles.
* Acyclovir, ribavirin, zidovudine, interferons are some of the important antiviral drugs, used these days.
(5) Antifertility Drugs :
“Chemcial substances which are used to check pregnancy in women are called anti-fertility drugs or birth control pills or oral contraceptives”.
*Birth control pills essentially contain a mixture of synthetic estrogen and progesteron derivatives. Both of these compounds are hormones.
eg. : Norethyndron, Ethynylestradiol (novestrol)
*Mifepristone is a synthetic steriod that blocks the effects of progesterone and is used as a “morning after pill” in many countries.
eg. : Mifepriston
4. Chemicals in Food
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Chemicals In Food
Chemicals are added to food for their preservation, enhancing their appeal and adding nutritive values in them Main catergories of food additives are as follows
(i) Food colours.
(ii) Flavours and sweeteners.
(iii) Fat emulsifiers and stabilising agents.
(iv) Flour improvers antistaling agent and bleaches.
(v) Antioxidants
(vi) Preservatives
(vii) Nutritional supplements such as minerals, vitamins and amino acids except category
None of the above have nutritive values.
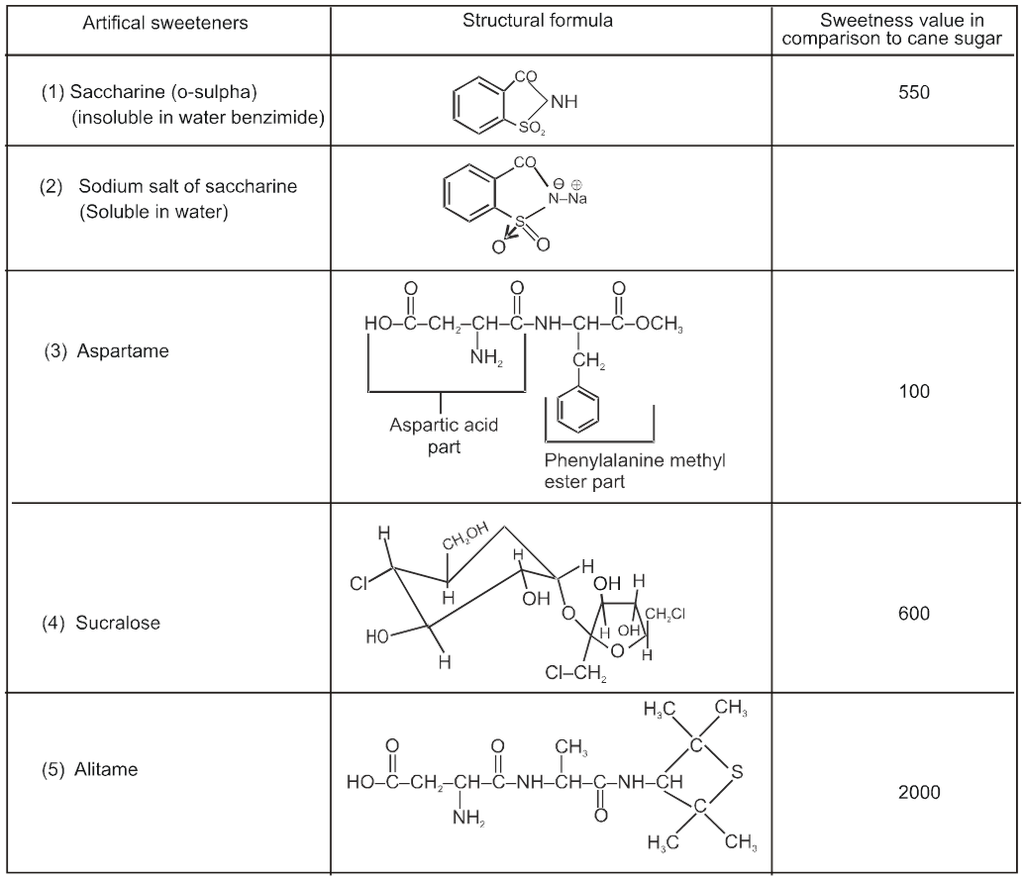
(a) Artificial sweetening agents :
* Saccharine is the first popular artificial sweetening agent used since 1879. It is about 550 times more sweet as cane sugar.
*It’s use is of great value to diabetic persons and people who need to control intake calories.
*It is used in pan masala cheap Ice cream, cheap drinks, mouthwash, cheap toffies, toothpaste).
(b) Food Preservatives :
*The chemical which are used to stop undesirable change in food caused by microorganism and save them from spoiling are called preservatives. It reduces (stop the growth) and rate reactions occuring due to bacteria in food).
*The following properties must be present in a preservative :
(i) It should not react with food material.
(ii) It’s effect should be for longer period.
(iii) It should not decrease the quality of food.
(iv) It should not have harmfull effect on the body.
Improtant preservatives are as follows :
(1) Sodium benzoate : It’s 0.06% to 0.1% concentration is used for preservation of fruit juice , jam, jelly, pickles etc.
(2) Parabens : These are alkyl p-hydroxy benzoate and used for preservation of tomato sauce etc.
(3) Sorbates : These are salt of sorbic acid and used for preservation of milk cheese preparation certain meats and fish products. It inhibit the growth of yeast
(4) Propionates : These are ethyl and phenyl ester of propionic acid and used for the preservation of biscuits and baked product, etc from mould fungi etc.
(5) Sodium or potassium metabisulphite (Na2S2O5 or K2S2O5) : It is used as a preservative for food products such as jams, squashes, pickles etc.
(6) Epoxides : Epoxides are gases and preserves low moisture foods like nuts, dried fruits. Epoxides destroy all type of microorganism including spores and viruses.
(7) p-Hydroxy benzoate ester : The methyl, ethyl propyl and heptyl esters of p-hyroxybenzoic acid are used as preservatives in baked foods, soft drinks, beer and syrups.
(c) Antioxidants :
*The chemical substance which reduce the rate of reaction with oxygen in food, thus help in their preservation are called antioxidants.
*They reduce the rate of formation of free radicals responsible for ageing process 2,6 ditertiary butylhydroxy toluene (p-crysol, BHT) and 2-tertiary butyl hydroxy anisole (BHA) are two most familiar antioxidants used.
5. Cleansing Agents :
Soaps and Detergents : *Soaps and detergents are used since long
SOAPS :
Soaps are sodium or potassium salts of long chain fatty acids e.g steric, oleic and palmitic acids. soap containg sodium salts are formed by heating fat (i.e. glyceryl ester of fatty acid) with aqueous sodium hydroxide solution. This reaction is known as SAPONIFICATION. Generally potassium soaps are soft to the skin.
Types of soaps :
There are so many types of soaps due to the using different raw materials
(i) Toilet soaps
(ii) Water floating soaps
(iii) Transparent soaps
(iv) Medicated soap
(v) Shaving soaps
(vi) Loundry soaps
(vii) Soaps chips
(viii) Soap granules
Detergents :
The synthetic products , which like soaps remove dust and grease from a surface are called detergents, since they are not soap but work like a soap so they are also called as soapless soap.
Detergents are mainly sodium salts of either sulphuric or sulphonic acids with long chain hydrocarbons.
eg. : Sodium alkyl sulphate or Sodium alkyl sulphonate
These can be used both in soft and hard water, as they give foam even in hard water
Synthetic detergents are mainly classified into three catagories :
(i) Anionic detergents : These are sodium salt of sulphonated long chain alcohols or hydrocarbons.
eg, : Lauryl alcohol, Lauryl hyrogen sulphate, Sodium lauryl sulphate
In anionic detergents, the anionic part of the molecule is involved in the cleansing action. These are smoothly used for household work and are also used in toothpastes.
(ii) Cationic detergents : These are quatenary ammonium salts of amines with acetates, chlorides or bromides as anion. Cetyltrimethylammonium bromide is a popular cationic detergent.
(iii) Non ionic detergents : These are moslty esters of poly hydroxy alcohols. They are in liquid form, and do not contain any ion in their constiution. One such detergent is formed when stearic acid reacts with polyethyleneglycol.
eg. : Stearic acid, Poly ethyleneglycol
Note : Liquid dish washing detergents are non ionic type.
Difference between soap and detergents
(1) Soaps are salts of weak acid and strong base whereas detergents are salts of strong acid and strong base.
(2) Aqueous solution of soap is basic where as aqueous solution of detergents is neutral.
(3) woolen and silk cloths in which soft fibres are present cannot be washed with soap whereas all type of fabrics can be washed with detergent
(4) Soap cannot work in hard water because soaps are precipitated as insoluble salt by reaction with Ca2+ and Mg2+ ions.
5. Rocket Propellant
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Rocket Propellant
* The propellants are chemical substances which on ignition provide thrust for the rocket to move forward. These substances are called rocket propellants.
(1) Types of rocket propellants : Depending upon the physical state, propellants can be classified as.
(i) Solid propellants : The solid propellants are mixtures of solid fuel and solid oxidiser. These are further divided into to classes.
(a) Composite propellants
(b) Double base propellants
(ii) Liquid propellants : These consist of an oxidizer such as liquid oxygen, nitrogen tetroxide (N2O4) or nitric acid and a fuel such as kerosene, alcohol, hydrazine or liquid hydrogen. These are further classified as,
(a) Monopropellants
(b) Bipropellants
Advantages of Biliquid Propellants over Solid Propellants
The biliquid propellants give higher thrust than solid propellants.
The thrust generated by liquid propellants can be controlled by switching on and off the flow of propellants. On the other hand, the thrust cannot be controlled in solid propellants.
(iii) Hybrid propellants : These are the propellants which consist of solid fuel and a liquid oxidiser. For
example, liquid N2O4 (liquid oxidiser) and acrylic rubber (solid fuel).
(2) Examples of Propellants used in Different Rockets
(i) Saturn booster rocket of American space programme used a mixture of kerosene and liquid oxygen as the propellant in the initial stage where as liquid oxygen and liquid hydrogen were used as propellant in high altitudes.
(ii) Russian rockets such as Proton used a liquid propellent consisting of kerosene and liquid oxygen.
(iii) The Indian satellites SLV-3 and ASLV used composite solid propellants.
(iv) The rocket PLSV will use solid propellant in the first and third stages and liquid propellant in second and fourth stages. The liquid propellant will consist of N2O4 and unsymmetrical dimethyl hydrazine (UDMH) and N2O4 and monomethyl hydrazine (MMH) respectively.
6. Nucleic acids
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Nucleic acids
Nucleic acids were first discovered by a Swiss biochemist, Friedrich Miescher (1869) who called them nuclein due to their acidic nature.Chemical analysis of chromosomes shows presence of two nucleic acids-DNA (Deoxyribo nucleic acid) and RNA (Ribo nucleic acid). Nucleic acid is a macromolecule & consists of many (polymer) monomeric units, called nucleotides. Each nucleotide is composed of a nucleoside and a phosphate group. Thus nucleotide is a phosphoric ester of nucleoside.
Each nucleoside consists of sugar molecule and a nitrogenous base. The relationship can be shown as given below.
Nucleic acid =many nucleotides
Nucleotide =nucleosides + phosphate
Nucleoside =sugar + nitrogenous base
Thus nucleotide =phosphate + sugar+ nitrogenous base
Nucleic acids bear different components that are briefly discussed below.
1. Phosphoric acid : The acidic nature of nucleic acids is due to the presence of phosphoric acid. Sugar of nucleoside combines with phosphoric acid by a phosphodiester bond formed at 5th or 3rd carbon of the sugar.
2. Sugar : It is a five carbon (pentose) sugar. There are two types of pentose sugars-ribose and deoxyribose. Deoxyribose sugar has one oxygen atom less at second carbon. Ribose sugar is present in RNA while deoxyribose sugar occurs in DNA.
3. Nitrogenous bases : they have two catagories
(a) Pyrimidine:- It includes cytosine, thymine and uracil. Pyrimidine bases are made of only one ring of carbon.
(b) purine:- It includes Adenine and guanine. Purine bases are made of two ring of carbon and nitrogen bases of DNA contains adenine, guanine, cytosine and thymine. In RNA, uracil is present in place of thymine.
Many nucleotide monomer units join one another to give rise to polynucleotide chain.
The two adjacent nucleotides are joined by formation of phosphodiester bond (a bond that involves two ester bonds). A polynucleotide chain is often written as 5’p 3’OH. This indicates that it is a dinucleotide with phosphate group (p) attached to the 5th carbon of terminal nucleotide and hydroxyl group (OH) is present at 3rd carbon of basal nucleotide.
Structure of DNA (Deoxyribonucleic acid) :
J.D. Watson and F.H.C. Crick (1953)
Proposed double helical structure of DNA based on the results of M.H.F.Wilkins and co-workers. All these three persons were awarded Nobel Prize in 1962 for this work.
The following are some of the characteristic features of double helical structure of DNA.
(i) Each nucleotide consists of sugar, phosphate and a nitrogenous base. Many such nucleotides are linked by phosphodiester bonds to form a polynucleotide chain or strand.
(ii) Phospho diester bonds are formed between 5’carbon of sugar of one nucleotide and 3' carbon of sugar of the next nucleotide.
(iii) Nitrogenous base is attached to1’ carbon of sugar. At this place purine base is attached by its 5' position and pyrimidine by its 3' position.
(iv) Polynucleotide strand is made of backbone of sugar and phosphate forming its long axis and bases at right angles to it.
Chargaff (1950)
Made observations on the base and other contents of DNA. These observations or generalizations are called Chargaffs rule.
(1) Purine and pyrimidine base pairs are in equal amount, that is, adenine + guanine =thymine + cytosine.
(2) Molar amount of purine-adenine is always equal to the molar amount of pyrimidine-thymine. Similarly, guanine is equalled by cytosine.
(3) Sugar deoxyribose and phosphate occur in equimolar proportions.
(4) The ratio of A + T/G + C is constant for a species.
(5) Chargaffs rule states that in natural DNAs the base ratio A/T is always close to unity and the G/C ratio also to always close to unity. It indicate that A always pairs with T and G pairs with C. A and T, G and C, therefore,are complementary base pairs.
(6) Thus, if one DNA strand has A, the other would have T and if it has G, the other, would have C. Therefore, if the base sequence of one strand is CAT TAG GAC, the base sequence of other strand would be GTA ATC CTG. Hence, the two poly nucleotide strands are called complementary to one another.
(7) Two such complementary strands are joined with each another by hydrogen bonds between their complementary nitrogenous bases. There are three hydrogen bonds between cytosine and guanine and two hydrogen bonds between adenine and thymine.
(8) The two polynucleotide chains are helically coiled around the same axis in such a way that these can separate from one another only by uncoiling. Helical coiling is supposed to be right handed. Such a form of DNA is now called B-DNA
(9) The two chains or strands are antiparallel, i.e., they run in opposite directions in relation to their sugar molecules. Their 5’p 3' OH phosphodiester linkages are in opposite directions
(10) Double stranded DNA molecule has a diameter of 20Aº.
(11) The helix makes one complete turn every 34 Aº along its length.
(12) There are 10 nucleotides per turn of helix.Thus the distance between two neighbouring base pairs is 3.4 A
RNA :
RNA is ribonucleic acid formed in the nucleus and is found in the cytoplasm of the cell.
Types of RNA and their functions :
(1) r-RNA (Ribosomal – RNA) :
It is found in the ribosomes and it is usually associated with protein to form the ribosomes. It is synthesised in the nucleus by DNA. It is single stranded, comprising about 80% of the total RNA. It is metabolically stable.
Functions : (i) This forms the site for protein synthesis.
(ii) It is also supposed to help the binding of m-RNA to the ribosomes during protein synthesis.
(2) m-RNA (Messenger RNA) :
It is carries the genetic message code from the D.N.A. to ribosomes. It is produced by the DNA ; m-RNA is also single stranded and constitutes about 15% of total RNA.
Functions : It carries the genetic information from DNA to the ribosomes where protein is synthesised.
(3) t-R.N.A (Transfer–RNA) :
It is synthesised in the nucleus by the DNA. It is also called soluble RNA. It is single stranded. There are 20 different kinds of t-RNA and each type has a specificity for a particular amino acid. It constitutes about 5% of total RNA. It has very short life.
Functions : It carries amino acids from different parts of cytoplasm to the ribosomes during protein synthesis.
7. Lipids
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Lipids
Lipids are constituents of plants and tissues which are insoluble in water but soluble in organic solvents such as chloroform, carbon tetrachloride, ether or benzene. They include large variety of compounds of varying structures such oils and fats; phospholipids, steroids, etc.
Lipids are mainly made up of carbon, hydrogen and oxygen. The number of oxygen atoms in a lipid molecule is always small as compared to the number of carbon atoms. Sometimes small amounts of phophsorus, nitrogen and sulphur are also present. They have a major portion of their structure like a hydrocarbon (aliphatic 663 or fused carbon rings).
Lipids serve as energy reserve for use in metabolism and as a major structural material in cell membranes for regulating the activities of cell and tissues.
Simple lipids are esters of glycerol with highly fatty acids (saturated or unsaturated). These are generally called glycerides of fats and oils.
Waxes are esters of fatty acids with certain alcohols, not glycerol. Fats and oils have biological importance, but waxes have no value as these are not digested.
Functions of triglycerides :
(i) They are energy reserves in the cells and tissues of living system. When digested, triglycerides are hydrolyzed to fatty acids and glycerol.
(ii) Catabolism of fatty acids form acetyl-coenzyme-A . Most of the energy of fatty acids is converted into ATP.
(iii) Acetyl co-enzyme is the starting material for the synthesis of many compounds.
(iv) Fats deposited beneath the skin and around the internal organs minimise loss of body heat and also act as cushions to absorb mechanical impacts.
Another very important class of lipids are the phospholipids. These are polar lipids and like the fats, are esters of glycerol. In this case, however, only two fatty acid molecules are-esterified to glycerol, at the first and second carbon atom. The remaining end position of the glycerol is esterified to a molecule of phosphoric acid, which in turn is also esterified to another alcohol. This gives a general structure.
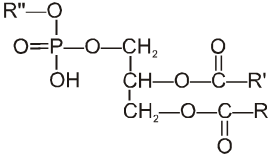
The alcoholic compound linked to phosphoric group may be ethanol, amine, serine or inositol. The phosphate group forms a polar end, i.e., hydrophilic (water-attracting) and the two fatty acid chains constitute ‘the non-polar tail, i.e., hydrophobic (water-repelling). This structure gives the phospholipids good emulsifying and membrane forming properties and hence they are used as good surfactants. Cell membranes are composed of phospholipids and proteins in about equal proportion.
The phospholipids in the membrane appear to be arranged in a double layer or bilayer in which the non-polar tails face each other, thereby exposing the polar heads to the aqueous environment on either side of the membrane. Proteins found in the membrane are embedded in the mossaic formed by the lipids.
Phospholipids facilitate the transport of ions and molecules in and out of the cell and regulate the concentration of molecules and ions within the cell. They provide structural support for certain proteins. The common examples of phospholipids are lecithins and cephanlins which are found in brain, nerve cells and liver of animals. These are also found in egg yolks, yeast, soyabeans and other foods.
Lecithins are derivatives of HOCH2CH2N(CH3)CI– and cephanlins ethanolamine, HOCH2CH2NH2•
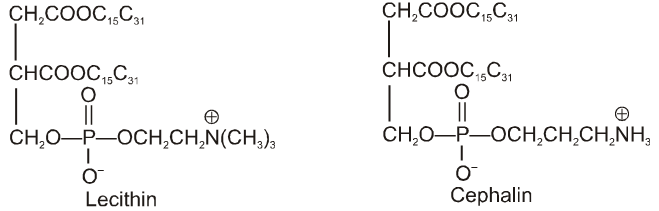
Lecithin contains a quaternary nitrogen whereas cephalin contains only primary nitrogen. The above mentioned lipids are mainly straight chain compounds. There is a third class of lipids which are not straight chain compounds. They are called Sterols. The sterols are composed of fused hydrocarbon rings and a long hydrocarbon side chain. Cholesterol is most important compound of this class and is found in animals only. It exists either free or as ester with a fatty acid. Cholesterol is also the precursor of hormones.
Cholesterol and its esters are insoluble in water. So they are deposited in the arteries and veins if the blood cholesterol rises. This leads to high blood pressure and heart diseases. Cholesterol is a part of animal cell membrane and is used to synthesise steroid hormones, vitamin-D and bile salts.
Waxes :
These are simple lipids and they are esters of long chain fatty acids and monohydric alcohols. General formula of waxes can be given as RCOOR’.
8. Enzymes and Hormones
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Enzymes and Hormones
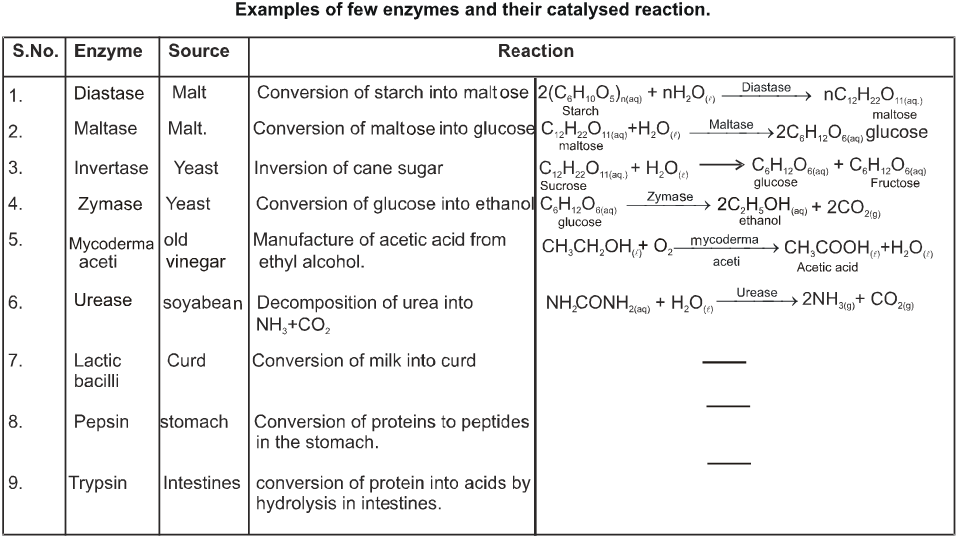
Enzymes :
Nitrogenous complex compounds with basic nature are called Enzymes. Enzymes are biological catalysts. All biological reactions are catalysed by enzyme. Enzyme lower the activation energy thereby increasing the rate of the reaction. For example activation energy for acid hydrolysis of sucrose is 6.22 KJ mol–1, while hydrolysis by SUCRASE enzyme the activation energy is 2.15 KJ mol–1.
Composition of enzymes :
Chemically all enzymes are globular proteins. However some enzymes are also associated with some non-protein component called the cofactor. For their activity. These cofactors are of two types : -
(a) Inorganic ions, such as Zn2+, Mg2+ , Mn2+ , Fe2+ , Cu2+ , Co2+ , Mo3+ , K+, Na+ etc
(b) organic molecules :- These are also of two types.
(i) Coenzymes : These are small organic molecules which are loosely held to protein and can be easily separated by dialysis. These are usually derived from vitamins such as thiamine, riboflavin, niacin etc.
(ii) Prosthetic group : These are also organic molecules which are tightly held to protein by covalent bonds but can be separated by only careful hydrolysis. Most of these are derived from vitamins such as biotin. Enzymes are vital for biological process without them the life process would be very slow. For example with out enzyme the meal would take 50 years to digest.
Characteristics of Enzymes :
(i) Specificity : Each enzyme catalyses only one chemical reaction.
(ii) Efficiency : Enzymes are very efficient catalyst. They increase the rate of reaction by 10 times.
(iii) Small quantity :- Only small amount of enzyme can be highly efficient because, they are regenerated after their catalytic activity
(iv) Optimum temp. and pH :- Enzymes catalyse reactions at pH of 7.4 and temperature range is 298-310 K (25ºC to 37ºC) (Exception pepsin work at pH 2–3)
Application of Enzymes :
(i) In the manufacture of BEER and WINE and vinegar by the fermentation of carbohydrates.
(ii) In the manufacture of sweet syrup from corn starch.
(iii) In the production of cheese by coagulation of milk.
(iv) Enzymes are helpfull mainly in the digestion process. Besides enzymes are used for controlling diseases such as
- Albinism diseases caused by the deficiency of the enzyme tryosinase
- streptokinase is used to dissolve blood clots in the treatment of heart disease.
Hormones :
These are the chemicals which are secreted by ductless glands and transported to different parts of the body by the blood stream where they control different physiological action of the body. The word hormone is derived from Greek language, which means to gear up or to excite. Hormones control cell and tissue growth, heart beat, blood pressure, secretion of digestive enzymes, kidney function, the reproductive system and lactation, etc. In mammals, the secretion of the hormones is controlled by anterior lobe of the pitutary gland located at the base of brain. These hormones are then carried to other gland such as adrenal cortex and sex glands to stimulate the production of other hormones.
Classification of hormones: On the basis of structure and composition, hormones are classified into following types:
(i) Steroid hormones:
These hormones are composed to steroid nucleus having following structure:
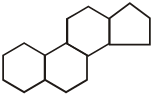
These are mostly secreted by Testes and adrenal cortex of males and hence, called as adrenal cortex hormones or sex hormones. Examples of these hormones are androgens Testosterones and dihydro testosterone. These hormones are stimulant of male sex characteristics. In females, sex hormones are estrogens. These hormones are produced in ovaries.
(ii) Polypeptides hormones :
These hormones are polypeptide (protein). These hormones are secreted by posterior lobe of the pitutary gland. eg. oxytocin, vasopressin.
(iii) Amine hormones :
These are water soluble hormones containing amino groups. These are derived from amino acids.
e.g thyroxine and adrenaline.
9. Vitamins
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Vitamins
Definition : Vitamins may be defined as a group of biomolecules which are required in small amounts for normal metabolic process and for the life, growth and health of human beings and animals.
Sources of Vitamins : Human body can synthesize vitamin eg. some members of vitamin B complex synthesized by micro organisms present in the intestinal tract. Most of the vitamins can not be synthesize by our body. Therefore, they must be supplied in the food. Plants can synthesize almost all vitamins. Vitamin D may either be supplied in the food or may be produced in the skin by the irradiation of ergosterol (a sterol present in our body) with ultraviolet light.
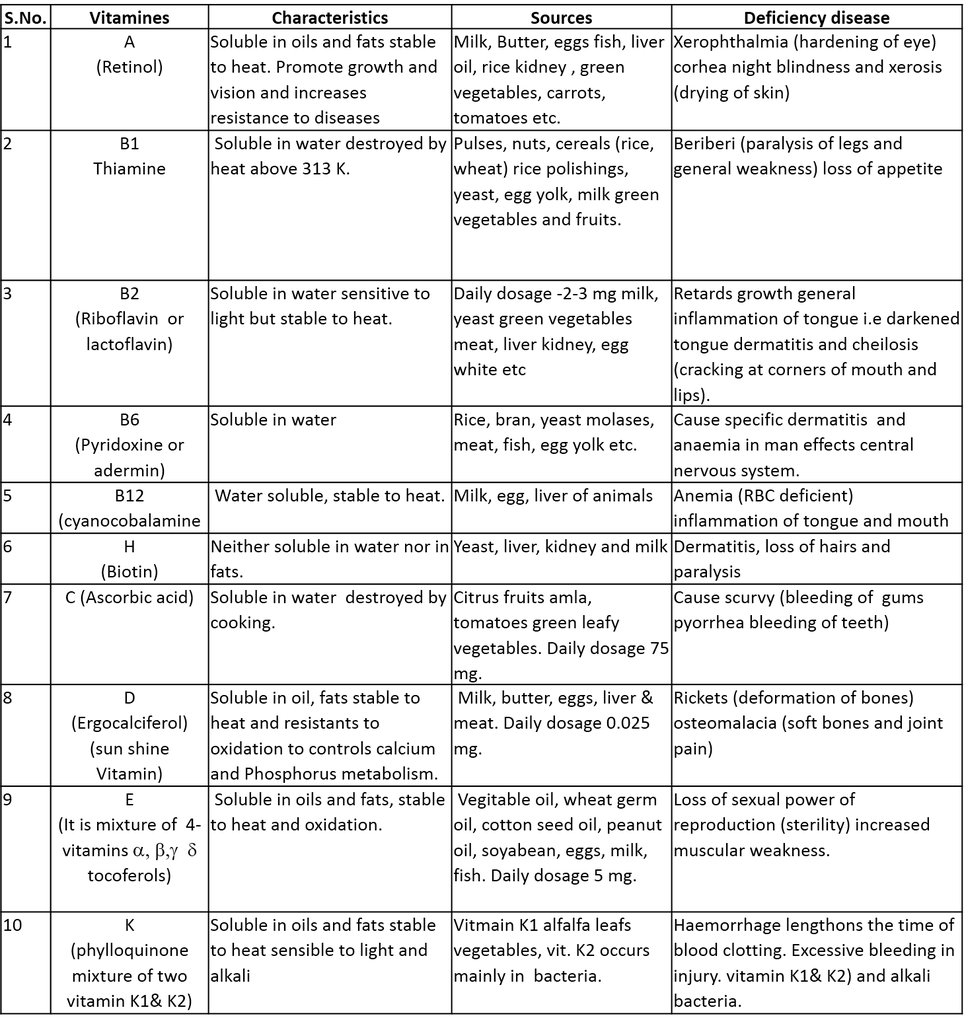
Classification of Vitamins : There is very little common to the various vitamins, therefore there are usually desiganated by alphabet letters A, B, C, D, E and K. Some of these are classified as sub groups eg. B1, B2, B3, B4, B6, B12 D1, D2 etc. About 25 vitamins are known to date. Vitamins are broadly classified into two catagories.
(i) Water soluble vitamins : Vitamin B-complex and vitamin C are water soluble vitamins and must be supplied regularly in diet.
(ii) Fat soluble vitamins : These are oily substances and soluble in fats These are A,D,E and K. They are stored in liver and adipose (fat storing) tissues.
(iii) Biotin (Vitamin H) : It is neither soluble in water nor in fats. Lack of particular vitamin causes a specific deficinecy disease. Some important vitamins their characteristics, sources and deficiency diseases are summarised in the following table.
10. Physical properties of organic compounds
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Physical properties of organic compounds
1. Bond Polarity :
A bond with electrons shared equally between the two atoms is called a nonpolar covalent bond.
Ex. Bond in H2 and the C–C bond in ethane are nonpolar covalent bonds.
Generally in most of the bonds between two different elements, the bonding electrons are attracted more strongly towards one of the two nuclei. An unequally shared pair of bonding electrons is called a polar covalent bond.
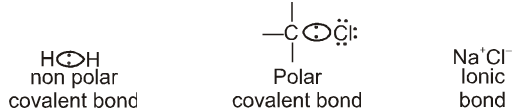
A covalent bond will be polar if atoms forming it differ in electronegativity.
Electronegativity F > O > Cl , N > Br > C,H
2. Intermolecular forces :
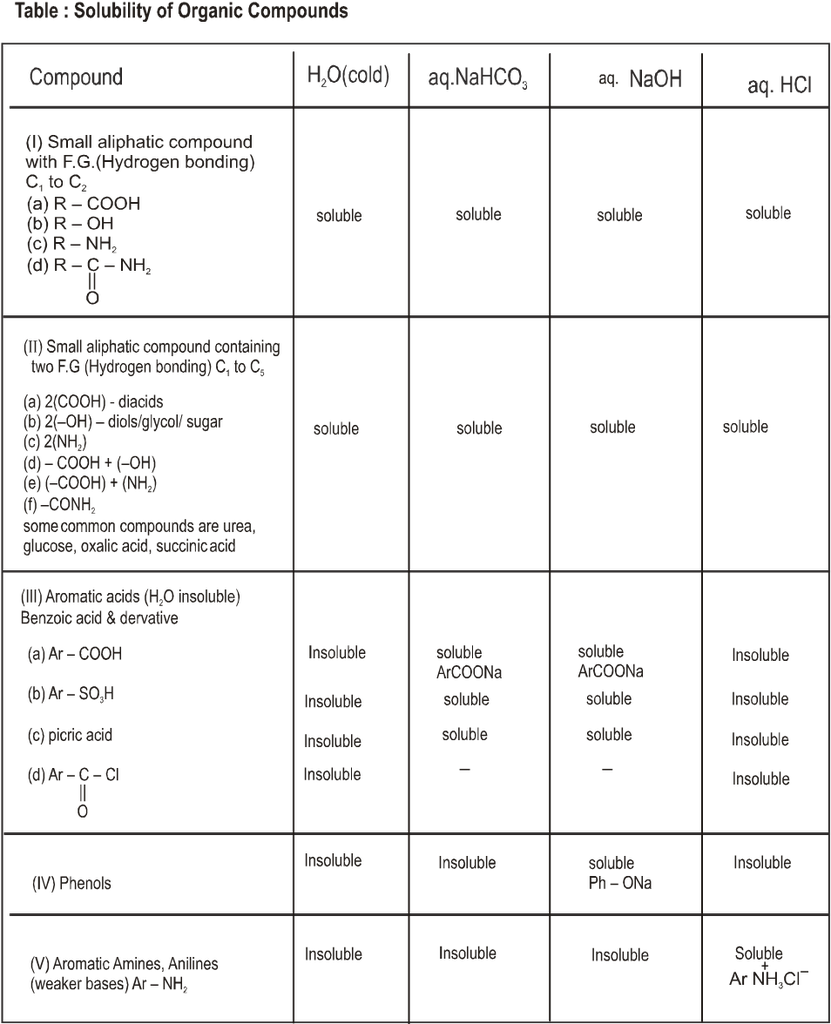
• Attractions between molecules are particularly important in solids and liquids. In these “Condensed” phases, the molecules are continously in contact with each other. The melting points, boiling points, and solubilities of organic compounds show the effects of these forces. Two major kinds of attractive forces cause molecules to associate into solids and liquids;
(A) Dipole-dipole interactions (B) VanderWaals forces
(A) Dipole-Dipole interaction :
Dipole-dipole interactions result from the approach of two polar molecules.
• If their positive and negative ends approach, the interaction is an attractive one.
• If two negative ends or two positive ends approach, the interaction is repulsive one.
• In a liquid or a solid the molecules are mostly oriented with the positive and negative ends together and the net forces is attractive.
Symbolized by :
![]()
• An especially strong kind of dipole- dipole attraction is hydrogen bonding
Types of Hydrogen Bonding :
(a) Intermolecular hydrogen bonding : In such type of linkages the two or more than two molecules of the same compound combine together to give a polymeric aggregate.
This phenomena is also known as association.
Example :
(I) Hydrogen bonding in carboxylic acids e.g. formic acid (Dimerisation)
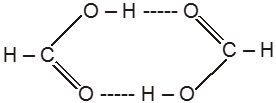
(II) In m-Chlorophenol
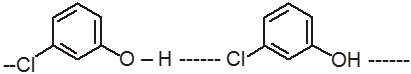
(III) In water
![]()
(IV) In p-Nitrophenol
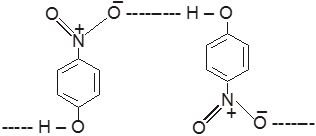
(V) In p-Nitrophenol & water
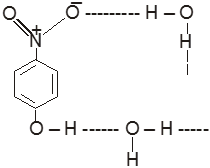
(b) Intramolecular hydrogen bonding:- In this type, hydrogen bonding occur within two atoms of the same molecule. This type of hydrogen bonding is commonly known as chelation.
Ex.
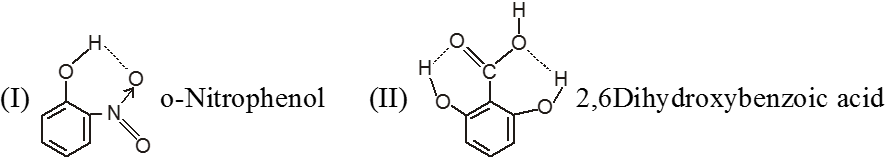
Conclusion :
(a) The chelation between the ortho substituted groups restricts the possibility of intermolecular hydrogen bonding.
(b) Chelation does not take place in m – & p – isomers because the two groups far away from each other.
3. Boiling Points :
• The boiling point (bp) of a compound is the temperature at which the compound’s vapor pressure equals the atmospheric pressure. In order for a compound to vaporize, the force that hold the molecules close to each other in the liquid must overcome. This means that the boiling point of a compound depends on the strength of the attractive forces between the individual molecules.
• If the molecules are held together by strong forces, it will take a lot of energy to pull the molecules away from each other and the compound will have a high boiling point.
• If however, the molecule are held together by weak forces, only a small amount of energy will be needed to pull the molecules away from each other and the compound will have a low boiling point.
Factors affecting boiling point :
(1) Hydrogen Bonding :
Alcohols :
Alcohols have much higher boiling points than alkanes or ethers of comparable molecular weight because, in addition to van der Waals forces and the dipole–dipole interactions of the carbon–oxygen bond alcohols can form hydrogen bonds.
The successive replacement of hydrogen atom of the –OH group of alcohol by alkyl group to form ether blocks the probability of hydrogen bonding reduces and thus B.P. of alcohols are higher than ether.
Ex. 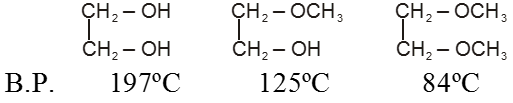
Water :
Water has the lowest molecular weight among hydrides of the VI group of periodic table, it has the highest boiling point. Water molecules associate through intermolecular hydrogen bonding and thus require more energy to separate the molecules for vaporization.
Ex. ![]()
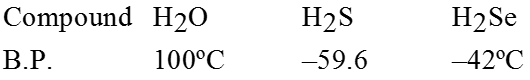
Amines :
• Primary and secondary amines also form hydrogen bonds, so these amines have higher boiling points than alkanes with similar molecular weights.
• Nitrogen is not as electronegative as oxygen, however, which means that the hydrogen bonds between amine molecules are weaker than the hydrogen bonds between alcohol molecules.
• Amines, therefore, have lower boiling points than alcohols with similar molecular weights.
• Because primary amines have two N–H bonds, hydrogen bonding is more significant for primary amines than for secondary amines. Tertiary amines cannot form hydrogen bonds with each other because they do not have a hydrogen attached to the nitrogen. Consequently if you compare amines with the same molecular weight and similar structures, primary amines have higher boiling point than secondary amines and secondary amines have higher boiling points than tertiary amines.
Ex.
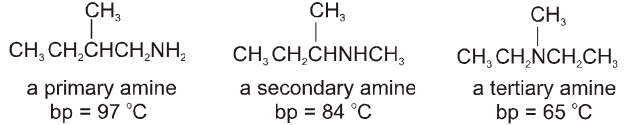
(2) Dipole - Dipole interactions :
Dipole–dipole interactions, are stronger than van der Waals forces but not as strong as ionic or covalent bonds. Ethers generally have higher boiling points than alkanes of comparable molecular weight because both van der Waals forces and dipole–dipole interactions must be overcome for an ether to boil.
Ex.

Ex. ![]()
(3) Molecular weight :
The boiling points for any homologous series of compounds increase as their molecular weights increase because of the increase in van der Waals forces. So the boiling points of a homlogous series of ethers, alkyl halides, alcohols, and amines increase with increasing molecular weight.
Ex. B. P : CH3 I > CH3Br > CH3 – Cl > CH3 – F
Ex..
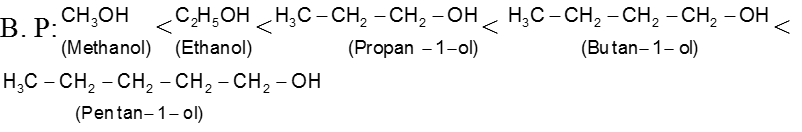
Ex.
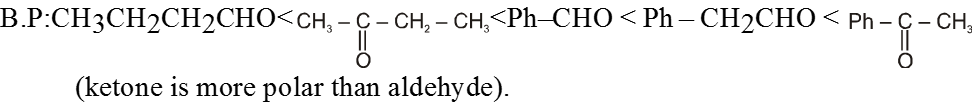
(4) Vander Waals forces
• The molecules of an alkane are held together by these induced dipole-induced dipole interactions known as van der Waals forces or London forces. In order for an alkane to boil, these van der Waals forces must be overcome.
• The homologous series of alkanes boiling points increase as their size increases. This occurs becuase each additional methylene group increases the area of contact between the molecules.
• Because the strength of van der Waals force depends on the area of contact between molecules, branching in a compound lowers its boiling point because it reduces the area of contact.
• If two alkanes have the same molecular weight, the more highly branched alkane will have a lower boiling point.
Ex.
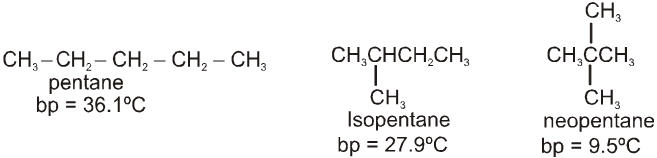
General order of boiling point of various F.G.
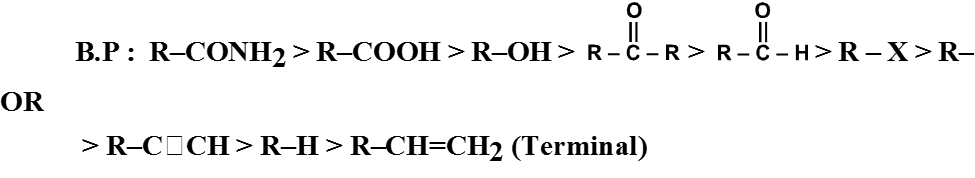
Ex. o-Chlorophenol has lower boiling point in comparison to its p-isomer.
![]() (Intramolecular H-bond in o-Chlorophenol)
(Intramolecular H-bond in o-Chlorophenol)
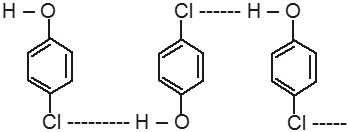 (Intermolecular H-bond in p-chlorophenol)
(Intermolecular H-bond in p-chlorophenol)
4. Melting Point :
The temperature at which the thermal energy of the particles is great enough to overcome the intracrystalline forces that hold them in position is known as melting point.
• Melting is the change from the highly orderd arrangment of particles in the crystalline lattice to the more random arrangment that characterizes a liquid.
Factors affecting melting point :
(1) Molecular weight :
Melting points of alkanes increase in a homologous series as the molecular weight increases.
Ex. M.P. : CH3–CH2–CH3 < CH3CH2–CH2–CH3 < CH3–CH2–CH2–CH2–CH3
(2) Packing :
• Packing is a property that determines how well the individual molecules in a solid fit together in the crystal lattice. If molecules are more closely packed then more energy is required to break the lattice and melt the compound.
• Alkanes with an odd number of carbon atoms pack less tightly than alkanes with an even number of carbon atoms. This decreases the intermolecular forces between alkanes with odd number of carbon atoms, which decreases their melting points.
• In geometrical isomers the trans isomers are more symmetrical than cis isomers (Cab=Cab type alkenes)
so trans form have higher M.P. than cis isomers.
- The heavier the molecule and stronger the intermolecular forces, higher will be the M.P. of the compound.
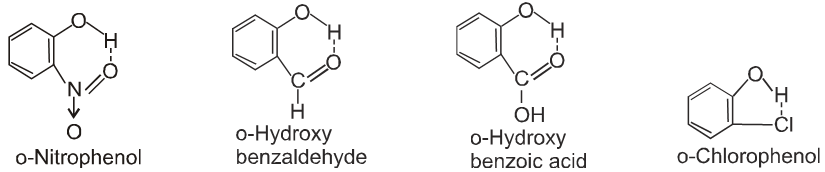
- Ortho isomers of hydroxy, nitro, carbonyl compounds have low M.P. than their corresponding m – & p – isomers.
Ex.
![]()
Ex. Ortho - hydroxy, nitro-, carbonyl, carboxylic or chloro compounds have lower melting and boiling points than the respective meta or para isomer due to interamolecular H-bonding in ortho substituted compound.
Ex. 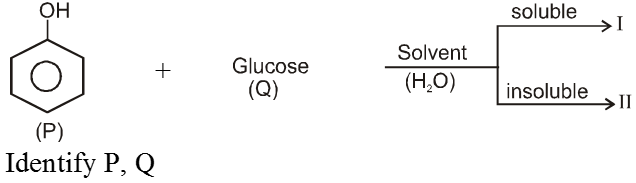
Sol. ![]()
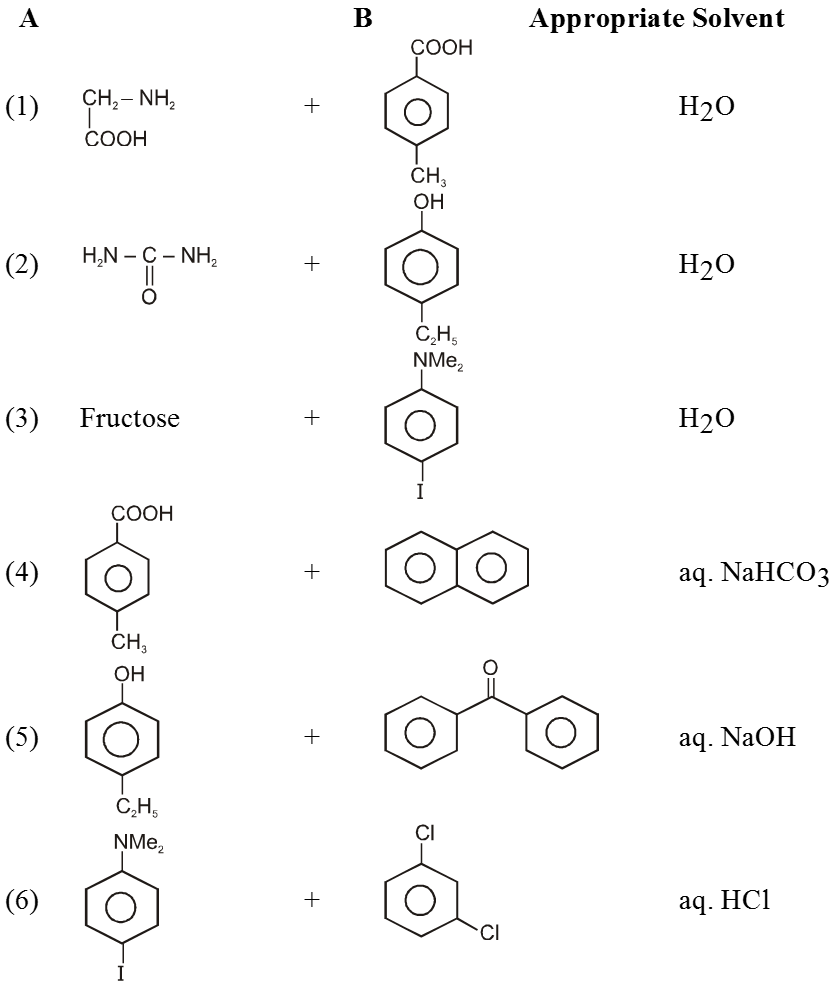
Ex. Binary mixtures - (Two components

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
