- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Chemicals In Food
Chemicals are added to food for their preservation, enhancing their appeal and adding nutritive values in them Main catergories of food additives are as follows
(i) Food colours.
(ii) Flavours and sweeteners.
(iii) Fat emulsifiers and stabilising agents.
(iv) Flour improvers antistaling agent and bleaches.
(v) Antioxidants
(vi) Preservatives
(vii) Nutritional supplements such as minerals, vitamins and amino acids except category
None of the above have nutritive values.
(a) Artificial sweetening agents :
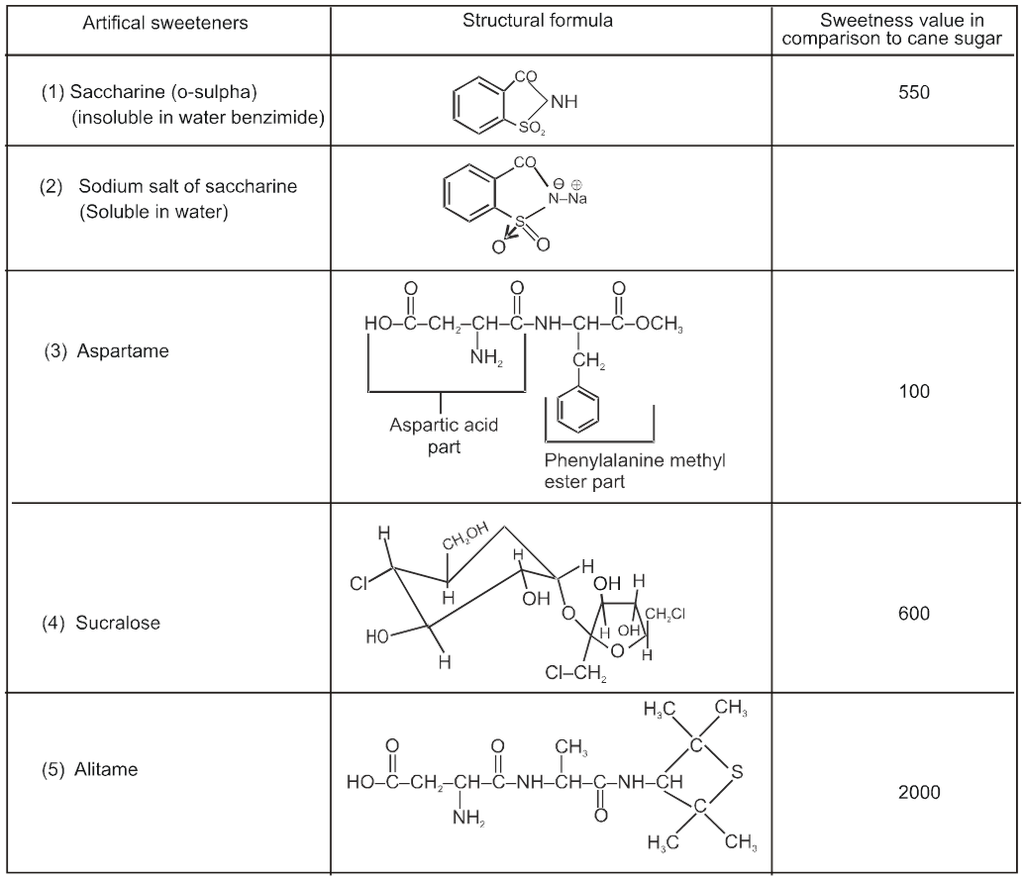
* Saccharine is the first popular artificial sweetening agent used since 1879. It is about 550 times more sweet as cane sugar.
*It’s use is of great value to diabetic persons and people who need to control intake calories.
*It is used in pan masala cheap Ice cream, cheap drinks, mouthwash, cheap toffies, toothpaste).
(b) Food Preservatives :
*The chemical which are used to stop undesirable change in food caused by microorganism and save them from spoiling are called preservatives. It reduces (stop the growth) and rate reactions occuring due to bacteria in food).
*The following properties must be present in a preservative :
(i) It should not react with food material.
(ii) It’s effect should be for longer period.
(iii) It should not decrease the quality of food.
(iv) It should not have harmfull effect on the body.
Improtant preservatives are as follows :
(1) Sodium benzoate : It’s 0.06% to 0.1% concentration is used for preservation of fruit juice , jam, jelly, pickles etc.
(2) Parabens : These are alkyl p-hydroxy benzoate and used for preservation of tomato sauce etc.
(3) Sorbates : These are salt of sorbic acid and used for preservation of milk cheese preparation certain meats and fish products. It inhibit the growth of yeast
(4) Propionates : These are ethyl and phenyl ester of propionic acid and used for the preservation of biscuits and baked product, etc from mould fungi etc.
(5) Sodium or potassium metabisulphite (Na2S2O5 or K2S2O5) : It is used as a preservative for food products such as jams, squashes, pickles etc.
(6) Epoxides : Epoxides are gases and preserves low moisture foods like nuts, dried fruits. Epoxides destroy all type of microorganism including spores and viruses.
(7) p-Hydroxy benzoate ester : The methyl, ethyl propyl and heptyl esters of p-hyroxybenzoic acid are used as preservatives in baked foods, soft drinks, beer and syrups.
(c) Antioxidants :
*The chemical substance which reduce the rate of reaction with oxygen in food, thus help in their preservation are called antioxidants.
*They reduce the rate of formation of free radicals responsible for ageing process 2,6 ditertiary butylhydroxy toluene (p-crysol, BHT) and 2-tertiary butyl hydroxy anisole (BHA) are two most familiar antioxidants used.
5. Cleansing Agents :
Soaps and Detergents : *Soaps and detergents are used since long
SOAPS :
Soaps are sodium or potassium salts of long chain fatty acids e.g steric, oleic and palmitic acids. soap containg sodium salts are formed by heating fat (i.e. glyceryl ester of fatty acid) with aqueous sodium hydroxide solution. This reaction is known as SAPONIFICATION. Generally potassium soaps are soft to the skin.
Types of soaps :
There are so many types of soaps due to the using different raw materials
(i) Toilet soaps
(ii) Water floating soaps
(iii) Transparent soaps
(iv) Medicated soap
(v) Shaving soaps
(vi) Loundry soaps
(vii) Soaps chips
(viii) Soap granules
Detergents :
The synthetic products , which like soaps remove dust and grease from a surface are called detergents, since they are not soap but work like a soap so they are also called as soapless soap.
Detergents are mainly sodium salts of either sulphuric or sulphonic acids with long chain hydrocarbons.
eg. : Sodium alkyl sulphate or Sodium alkyl sulphonate
These can be used both in soft and hard water, as they give foam even in hard water
Synthetic detergents are mainly classified into three catagories :
(i) Anionic detergents : These are sodium salt of sulphonated long chain alcohols or hydrocarbons.
eg, : Lauryl alcohol, Lauryl hyrogen sulphate, Sodium lauryl sulphate
In anionic detergents, the anionic part of the molecule is involved in the cleansing action. These are smoothly used for household work and are also used in toothpastes.
(ii) Cationic detergents : These are quatenary ammonium salts of amines with acetates, chlorides or bromides as anion. Cetyltrimethylammonium bromide is a popular cationic detergent.
(iii) Non ionic detergents : These are moslty esters of poly hydroxy alcohols. They are in liquid form, and do not contain any ion in their constiution. One such detergent is formed when stearic acid reacts with polyethyleneglycol.
eg. : Stearic acid, Poly ethyleneglycol
Note : Liquid dish washing detergents are non ionic type.
Difference between soap and detergents
(1) Soaps are salts of weak acid and strong base whereas detergents are salts of strong acid and strong base.
(2) Aqueous solution of soap is basic where as aqueous solution of detergents is neutral.
(3) woolen and silk cloths in which soft fibres are present cannot be washed with soap whereas all type of fabrics can be washed with detergent
(4) Soap cannot work in hard water because soaps are precipitated as insoluble salt by reaction with Ca2+ and Mg2+ ions.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
