- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Isomerism in coordination compounds :
(1) Structural isomerism :
The structural isomerism arises due to different lignads within the coordination sphere.
(A) Ionisation Isomerism
This type of isomerism occurs when the counter ion in a coordination compound is itself a potential ligand and can displace a ligand which can then become the counter ion. For example, following complexes show ionisation isomerism.
[Co(NH3)5SO4]NO3 and [Co(NH3)5NO3]SO4
[Co(NH3)4(NO2)Cl]Cl and [Co(NH3)4Cl2]NO2.
[Co(NH3)4(H2O)Cl]Br2 and [Co(NH3)4BrCl]Br.H2O. [Also an example of hydrate isomers.]
[Pt(NH3)4Cl2]Br2, and [Pt(NH3)4Br2]Cl2.
[CoCl(en)2(NO2)]SCN, [Co(en)2(NO2)SCN]Cl and [Co(en)2(SCN)Cl]NO2
(B) Solvate/Hydrate Isomerism :
It occurs when water forms a part of the coordination entity or is outside it. This is similar to ionisation isomerism. Solvate isomers differ by whether or not a solvents molecule is directly bonded to the metal ion or merely present as free solvent molecules in the crystal lattice. For example, CrCl3 . 6H2O exists in three distinct isomeric forms : [Cr(H2O)6]Cl3, violet ; [CrCl(H2O)5]Cl2.H2O, blue green : [CrCl2(H2O)4]Cl.2H2O, dark green.
(C) Linkage isomerism :
In some ligands, like ambidentate ligands, there are two possible coordination sites. In such cases, linkage isomerism exist. e.g., NO2 group can be bonded to metal ions through nitrogen (–NO2) or through oxygen (–ONO). SCN too can be bonded through sulphur (–SCN) thiocyanate or through nitrogen (–NCS) isothiocyanate.
For example : [Co ONO (NH3)5] Cl2 & [Co NO2 (NH3)5] Cl2 .
(D) Coordination Isomerism
Coordination compounds made up of cationic and anionic coordination entities show this type of isomerism due to the interchange of ligands between the cation and anion entities. Some of the examples are :
(i) [Co(NH3)6][Cr(CN)6] and [Cr(NH3)6](Co(CN)6]
(ii) [Cu(NH3)4](PtCl4] and [Pt(NH3)4][CuCl4]
(iii)[Co(NH3)6][Cr(SCN)6]and[Cr(NH3)4(SCN)2](Co(NH3)2(SCN)4]
(iv) [Pt(NH3)4][PtCl6] and [Pt(NH3)4Cl2][PtCl4]
Such isomers are expected to have significant differences in their physical and chemical properties.
(2) Stereoisomerism
The isomers in which atoms are bonded to each other in the same order but that differ in the arrangement of these atoms in the space are called as stereoisomers and the phenomenon as stereoisomerism.
(A) Geometrical Isomerism
This type of isomerism arises in heteroleptic complexes due to different possible geometric arrangements of the ligands. Geometrical isomerism is common among coordination compounds with coordination numbers 4 and 6.
(i) Coordination Number Four :
(a) Tetrahedral Complex :
The tetrahedral compounds can not show geometrical isomerism as we all know that all four positions are equivalent in tetrahedral geometry.
(b) Square Planar Complex :
In a square planar complex of formula [Ma2b2] [a and b are unidentate], the two ligands ‘a’ may be arranged adjacent to each other in a cis isomer, or opposite to each other in a trans isomer as depicted.
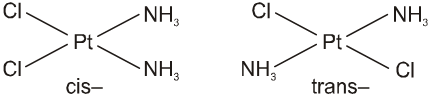
Geometrical isomers (cis and trans) of Pt(NH3)2Cl2 .
Square planar complex of the type Ma2bc (where a,b,c are unidentates) shows two geometrical isomers.

Square planar complex of the type Mabcd (where a,b,c,d are unidentates) shows three geometrical isomers.

Example is [Pt(NH3)BrCl(py)]. three isomers of the complex [Pt(NH3)(NH2OH)(py)(NO2)]+ have been isolated and identified.
Square planar complex of the type M(AB)2 (where AB are unsymmetrical bidentates) shows two geometrical isomers. Example is [Pt(gly)2] in which gly is unsymmetrical ligand.
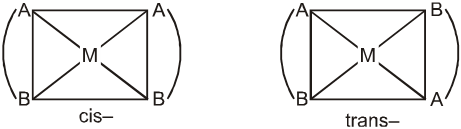
Similarly, M(AB)(CD) also shows two geometrical isomers.
Note : M(AA)2, (where AA are symmetrical bidentates) does not show geometrical isomerism. e.g., [Cu(en)2]2+ [Pt(ox)2]2–, etc.
(ii) Coordination Number Six :
Geometrical isomerism is also possible in octahedral complexes.
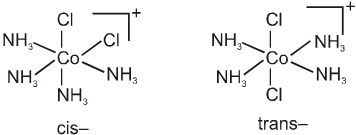
Geometrical isomers (cis and trans) of [Co(NH3)4Cl2]+
Number of possible isomers and the spatial arrangements of the ligands around the central metal ion for the specific complexes are given below.
(I) Complexes containing only unidentate ligands
(a) Ma2b4 – 2
(b) Ma4bc – 2
(c) Ma3b3
Complexes of the formula Ma3b3, where a and b are monodentate ligands, may show two isomeric forms called fac– and mer–. Facial isomers have three identical ligands on one triangular face where as meridional isomers have three identical ligands in a plane bisecting the molecule. Similar isomers are possible with some chelating ligands.
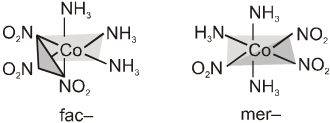
The facial(fac) and meridional(mer) isomers of [Co(NH3)3(NO2)3].
Unsymmetrical bidentate ligands also show fac-mer isomerism
(d) Ma3b2c – 3
(e) Ma3bcd – 4
(f) Ma2b2c2 – 5
(g) Ma2b2cd – 6
(h) Ma2bcde – 9
(i) Mabcdef, [Pt(py)(NH3)(NO2)(Cl)(Br)(I)] – 15
(II) Compounds containing bidentate ligand and unidentate ligands.
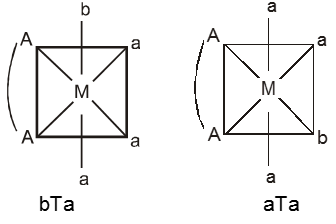
(i) M(AA)a3b – Two geometrical isomers are possible.
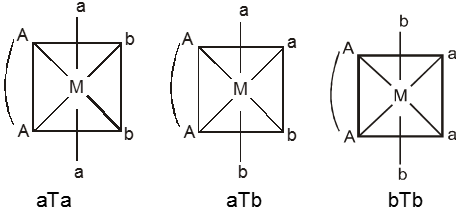
(ii) M(AA)a2b2 – Three geometrical isomers are possible.
(B) Optical Isomerism :
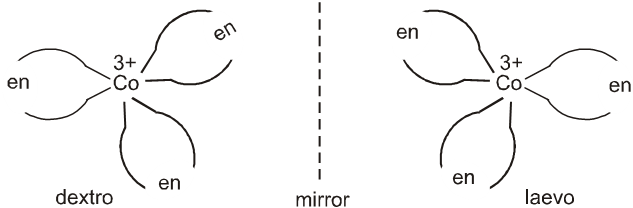
A Coordination compound which can rotate the plane of polarised light is said to be optically active. When the coordination compounds have same formula but differ in their ability to rotate directions of the plane of polarised light are said to exhibit optical isomerism and the molecules are optical isomers. Optical isomers are mirror images that cannot be superimposed on one another. These are called as enantiomers. The molecules or ions that cannot be superimposed are called chiral. This is due to the absence of elements of symmetry in the complex. The two forms are called dextro(d) and laevo(l) depending upon the direction they rotate the plane of polarised light in a polarimeter (d rotates to the right,l to the left).
(a) Octahedral complexes :
Optical isomerism is common in octahedral complexes involving didentate ligands. For example, [Co(en)3]3+ has d and l forms as given below
Cis-isomer of [PtCl2(en)2]2+ show optical isomerism as shown below because of the absence of plane of symmetry as well as centre of symmetry.
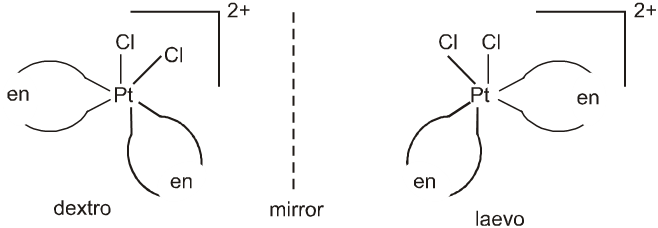
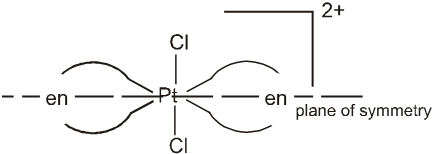
But trans isomer of [PtCl2(en2)]2+ does not show optical isomerism.
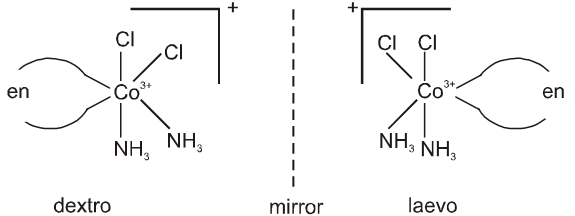
cis-[Co(NH3)2Cl2(en)]+ can show optical isomerism due to the absence of plane of symmetry as well as centre of symmetry.
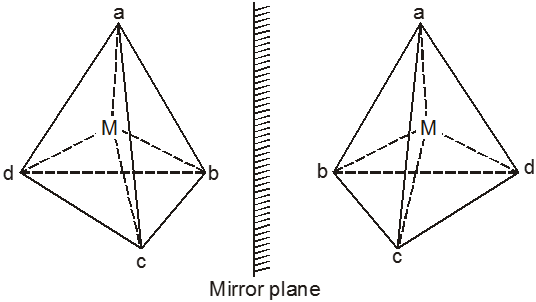
(b) Tetrahedral Complexes.
Optical isomerism is expected in tetrahedral complexes of the type [Mabcd] analogous to tetrahedral carbon atom.
(c) Square Planar Complexes.
Square planar complexes are rarely found to show the optical isomerism. The plane formed by the four ligating atoms and the metal ion is considered to be a mirror plane and thus prevents the possibility of chirality.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
