- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Chapter 11: Reaction Mechanism
Introduction :
Alkyl halides :
There are three major classes of organohalogen compounds ; the alkyl halides, the vinyl halides, and the aryl halides.
An alkyl halide simply has a halogen atoms bonded to one of the sp3 hybrid carbon atoms of an alkyl group. (A vinyl halide or Aryl halide has a halogen atom bonded to one of the sp2 hybrid carbon atoms of an aromatic ring. They are different from alkyl halides because their bonding and hybridization are different.)
Classification of halides :
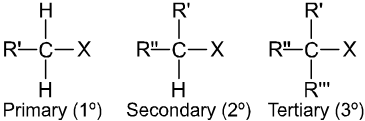
(a) Alkyl halides or haloalkanes (R—X) Compounds Containing sp3 C–X Bond :
They are classified as primary, secondary or tertiary according to the nature of carbon to which halogen is attached.
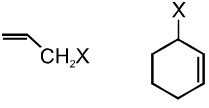
(b) Allylic halides :
These are the compounds in which the halogen atom is bonded to an sp3–hybridised carbon atom next to carbon-carbon double bond (C=C) i.e. to an allylic carbon.
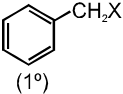
(c) Benzylic halides :
These are the compounds in which the halogen atom is bonded to an sp3–hybridised carbon atom next to an aromatic ring.
(d) Compounds Containing sp2 C–X Bond : Vinylic halides ![]() Aryl halides
Aryl halides ![]()
Structure of alkyl halide :
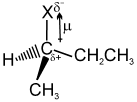
The carbon-halogen bond in an alkyl halide is polar because halogen atoms are more electronegative than carbon atoms. Most reactions of alkyl halides result from breaking this polarized bond. The carbon atom has a partial positive charge, making it some what electrophilic.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
