- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 12
- Subject
- Chemistry
Denticity and Chelation :
Table : 1
Common Monodentate Ligands
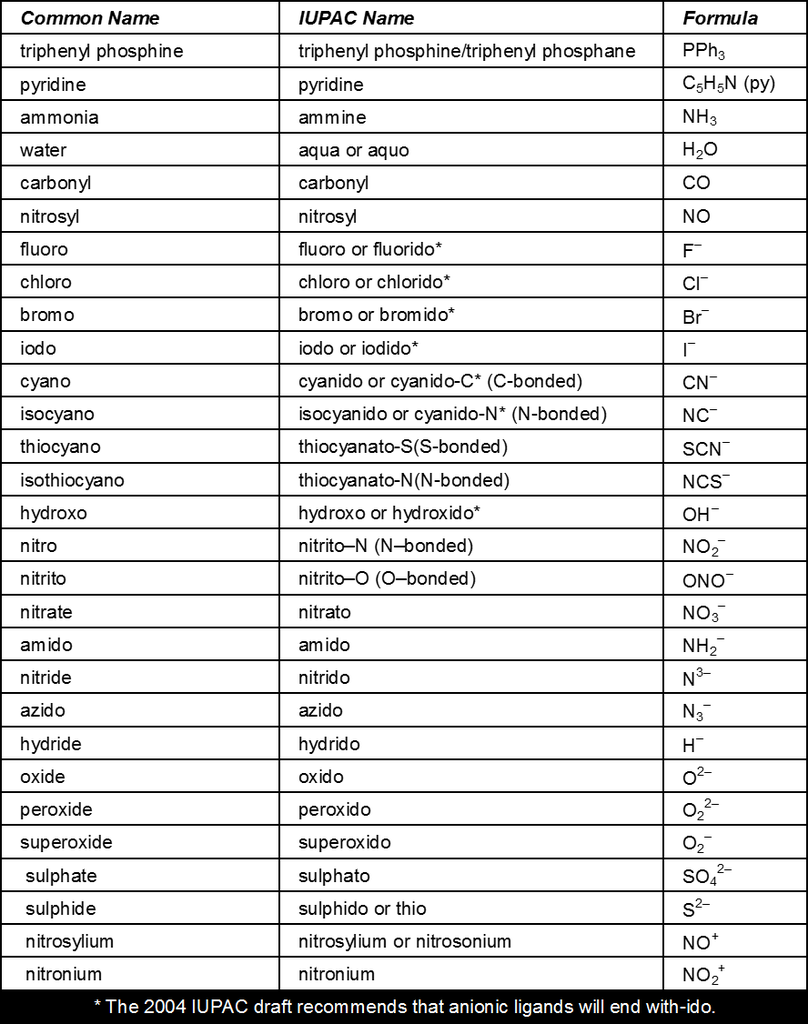
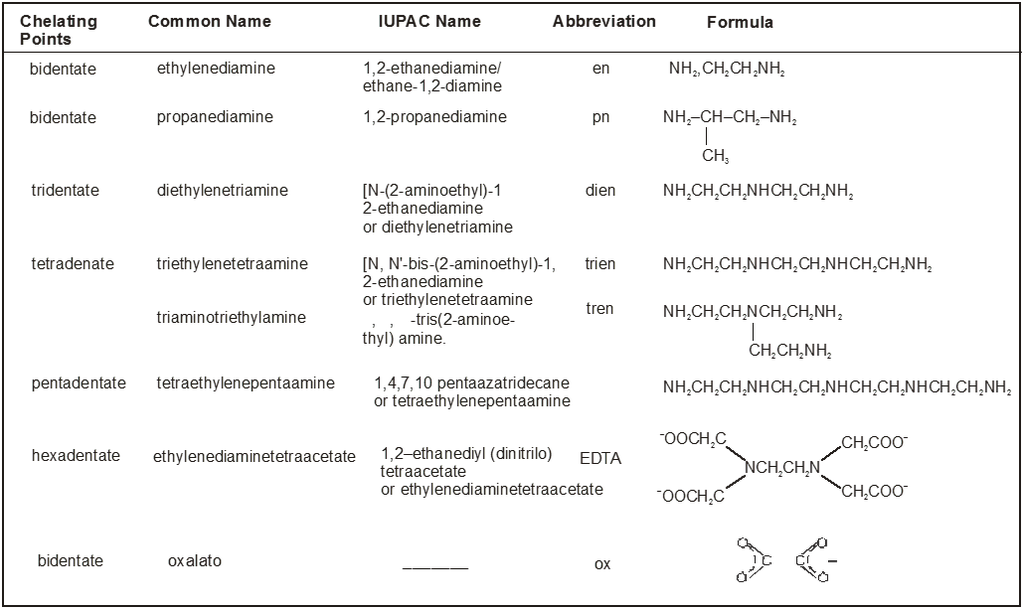
Table : 2
Common Chelating Amines
Coordination Number :
The coordination number of the central atom/ion is determined by the number of sigma bonds between the ligands and the central atom/ions i.e. the number of ligand donor atoms to which the metal is directly attached. Pi-bonds, if any, between the ligating atom and the central atom/ion are not considered for the determination of coordination number. The sigma bonding electrons may be indicated by a pair of dots, preceding the donor atom in the ligand formula as in :
[Co(NH3)6]3+, [Fe(CN)6]3–, [Ni(CO)4], [Co(Cl4)]2–.
Some common co-ordination number of important metals are as given below.

Coordination Polyhedron :
The spatial arrangement of the ligand atoms which are directly attached to the central atom/ion defines a coordination polyhedron about the central atom. Figure below shows the shapes of tetrahedral, square planar, octahedral, square pyramidal and trigonal bipyramidal coordination polyhedra. [Co(NH3)6]3+ has an octahedral geometry, while [PtCl4]2– and Ni(CO)4, are square planar and tetrahedral, respectively.
Oxidation number of Central Atom :
The oxidation number of the central atom is defined as the charge it would carry if all the ligands are removed along with the electron pairs that are shared with the central atom. Metal oxidation number is represented by a Roman numeral in parentheses following the name of the coordination entity. For example oxidation number of iron in [Fe(CN)6]3– is +3 and it is written as Fe(III).
Homoleptic and heteroleptic complexes
Complexes in which a metal is bound to only one type of donor groups, e.g., [Cr(NH3)6]3+, are known as homoleptic. Complexes in which a metal is bound to more than one type of donor groups. e.g., [Co(NH3)4Br2]+, are known as heteroleptic.

 ACME SMART PUBLICATION
ACME SMART PUBLICATION
