Periodic Classification of Elements
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Chapter:- 5
Periodic Classification of Elements
Periodic Classification of Elements
Elements are the building blocks of all Substances. There are 118 elements known at present, out of which 98 are naturally occurring. In this chapter, we will study various attempts which have been made to classify the elements from time to time. And finally we will study the modem classification of elements.
Attempts Earlier to Mendeleev
Several attempts have been made to classify elements. We important of which are discussed.
- Lavoisier’s Classification (1789)
- Debereiner's Triads (1817)
- Newlands' Law of Octaves
Lavoisier’s Classification (1789)
It is one of the earliest attempts to classify elements. Lavoisier divided elements into two main divisions, which arc called metals and non-metals.
Debereiner's Triads (1817)
In the year 1817, Johann Wolfgang Dobereiner, a German chemist; identified some groups having three elements each with similar properties. So, he called these groups as triads. When the three elements in a triad were arranged in the order of increasing atomic masses, the atomic mass of the middle element was roughly the average of the atomic masses of the other two elements.
For Example
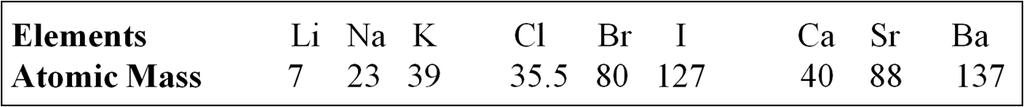
![]()
Newlands' Law of Octaves
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Newlands' Law of Octaves
In 1866. John Newlands, an English scientist; arranged the kown elements in the order of increasing atomic masses' and found that every eighth element had properties similar to that of the first. He called it the law of octaves. This is known as Newland’s law of octaves.
For Example
Newlands’ Octaves
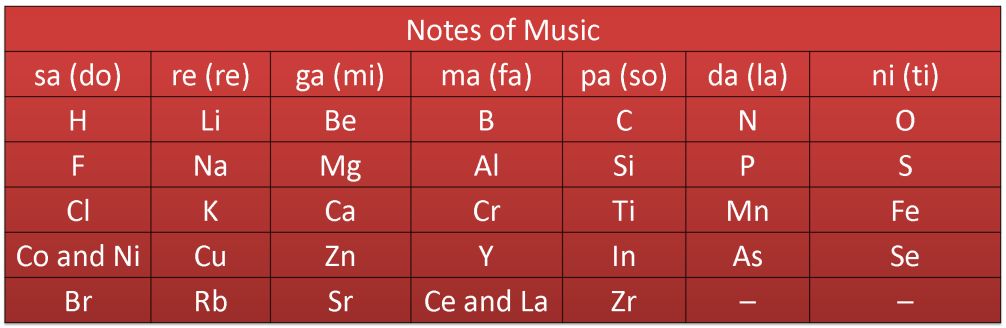
Application
It clearly represented that there was some systematic relationship between the atomic masses and repetition of properties of elements.
Limitations
- This law was successful only up to calcium. After that every eight element did not possess the same properties as those possessed by the elements lying above it in the same group.
- Newlands thought that there were only 56 elements that existed in nature and no more elements would be discovered in the future.
- In order to fit clement into his table, Newlands' adjusted two elements in the same slot, and also put some unlike elements under the same slot.
For Example, cobalt and nickel placed in the same column as fluorine, chlorine and bromine which have very different properties then these elements Iron, which resembles cobalt and nickel in properties, had been placed far away from these elements.
Mendeleev's Periodic Table (1834 – 1907)
Dmitri Ivanovich Mendeleev, a Russian chemist, was the most important contributor for the development of periodic table. 63 elements were known at the Mendeleev's time.
To classify elements
- He examined the relationship between the atomic masses of the elements and their physical and chemical properties.
- Among chemical properties, he concentrated on the compounds formed by the elements with oxygen and hydrogen.
- He took the formulae of the hydrides and oxides formed by an element as one of the basic properties.
Mendeleev's Periodic Law
On this basis Mendeleev formulated a periodic law, which states, “the physical and chemical properties of elements are the periodic function of their atomic masses.”
Features of Mendeleev's Periodic Table
The important features of this table are
- This table contains 8 vertical columns, called groups and 6 horizontal rows, called periods.
- It contains gaps for the elements not discovered at that time. He named such elements by prefixing a Sanskrit numeral eka (one), divi (two), tri (three), etc, to the name of the preceding similar (analogous) clement in the Same group.
For Example eka-boron, eka-silicon, which after their discovery were named as scandium, gallium and getmanium respectively.
- He also predicted the atomic masses and properties of several elements that were not known at that time.
- In order to group the elements having similar properties together, at some places. Mendeleev had to place an element with a slightly greater atomic mass before an element with a slightly lower atomic mass.
- With the help of this table, Mendeleev corrected atomic masses of some elements.
For Example, he corrected the atomic mass of be from 13 to 9.
Limitations of Mendeleev's Periodic Table
This table was greatly helpful for the study of elements but a few anomalies could not be explained on the basis of the table.
These anomalies are
- Position of Hydrogen
- Position of Isotopes
- Uncertainty in Atomic Masses
- Placing of Heavier Element before the Lighter One
Position of Hydrogen
In outer electronic configuration, hydrogen resembles with alkali metals, but tuft like halogen it exists in diatomic form and combines with metals and non-metals to form covalent compounds. Thus, its position was not fixed. But it was kept with alkali metals.
Position of Isotopes
Isotopes are the elements, having similar chemical properties but different atomic masses. In Mendeleev's periodic table, no place was given to these elements"
Uncertainty in Atomic Masses
Another problem was that the atomic mass do not increase in a regular manner in going from one, element to the next. So, it was not possible to predict how many elements could be discovered between two elements especially when we consider the heavier elements.
Placing of Heavier Element before the Lighter One
Few elements, those possess higher atomic mass were placed before elements having a lower atomic mass.
For Example, Argon (39.9) was placed before potassium 39.1, Cobalt (58.9) before nickel (58.6), tellurium (127.60) before iodine (126.9).
Modern Periodic Table and Various Trends in it
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Modern Periodic Table and Various Trends in it
In 1913. Henry Moseley showed that the atomic number of an element is' a more fundamental property and on the basis of this, he modified the Mendeleev's periodic law as “Physical and chemical properties of the elements are a periodic function of their atomic number”. This is called modern periodic law.
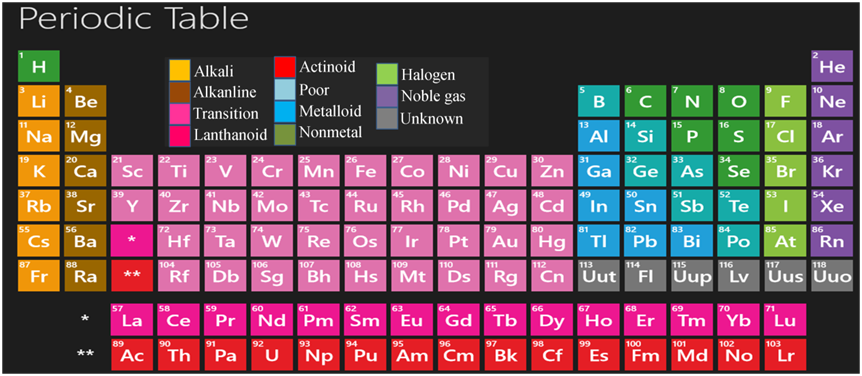
Features of Modern Periodic Table
This table has 18 vertical columns, known as groups and 7 horizontal rows, known as periods.
Features of Groups
- A few important features of the elements present in groups are as follows.
The elements present in a group are separated by definite gaps of atomic numbers.
- The groups are not divided into sub-groups.
- The elements present in a group have the same number of electrons in the valence shell of their atoms or same outermost electronic configuration.
- The elements present in a group have the same valiancy.
- The elements present in a group have identical chemical properties.
- The number of shells increases as we go down the group.
- The elements present in the group 18 are almost chemically uncreative. These are also called noble gas elements.
Features of Periods
The important features of the elements in a period are as follows:-
- Elements of a period do not have the same number of valence electrons but they contain the same number of shells.
- The number of valence shell electrons increase by one unit as the atomic number increases by one unit on moving from left to right in a period.
- As the number of valence shell electrons changes, the chemical properties of the elements also change
- Different periods have different number of electrons which can be explained on the basis of filling of electrons into various shells.
Position of the Elements in the Modern Periodic Table
- In order to find the position of an element in the periodic table, first write its electronic Configuration and then find period and group number as follows.
- The period number of an element is equal to the number of electron shells in its atom.
For Example, if the atom of an element has 2 electron shells (K and L), then it belongs to 2nd period.
- If two (or more) elements have the same number of valence shells, then they belong to the same period of the periodic table.
- The group number of an element having up to two valence electrons is equal to the number of valence electron.
Explanation of the Anomalies of Mendeleev's Periodic Table
Different anomalies of Mendeleev’s table can be explained with the help of modern periodic table as
- The fundamental basis for modern periodic table is atomic number, not atomic mass, hence it is more accurate.
- Since, the table is based on atomic number and isotopes have same atomic number and chemical properties, so they can be put at one place in the same group of the periodic table.
- In this periodic table, a unique, position has been given to the hydrogen. It is kept at the top left corner because of its unique characteristics.
- The position of cobalt and nickel is justified itself because atomic number of cobalt is less than atomic number of nickel.
- A separate group for noble gases was created when these were discovered.
Trends in the Modern Periodic Table
In this table, some properties show a regular trend when we move along a period from left to right or in a group from top to bottom.
These properties are explained in next topics
Valency
It is the combining capacity of an atom of an element to acquire noble gas configuration and depends upon the number of valence electrons the electrons present in the outermost shell.
Variation Along a Group
In a group, outer electronic configuration is same for all the elements, so all have the same number of valence electrons or the valency.
e.g. in case of elements of group 1
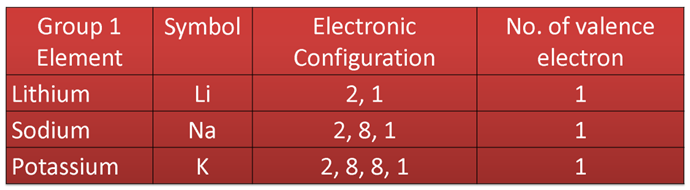
Similarly, for the elements of group 17

Variation Along a Period
Since, the number of valence electrons increases one by one from left to right along a period, so valency also increases from 1 to 7 with respect to oxygen. While with respect to hydrogen it first increases from 1 to 4 and then decreases to 0.

Atomic Size
It refers to the radius of an atom and is defined as the distance between the centre of the nucleus and the outermost shell of an isolated atom.
Variation Along a Period
The atomic radius decreases on moving from left to right along a period. This is due to an increase in nuclear charge which tends to pull the valence electrons closer to the nucleus and reduces the size of the atom
Variation Along a Group
The atomic size increases down the group. This is because new shells are being added as we go down the group. This increases the distance between the outermost electrons and the nucleus.
Metallic and Non-metallic Properties
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Metallic and Non-metallic Properties
Elements, having a tendency to lose one or more electrons and form positive ions are called metals. These are present on the left side as well as in the centre of the periodic table. Because of the formation of positive ions, these arc also called electropositive elements.
Non-metals are the elements which have a tendency to gain one or more electrons to form negative ions. Thus, these are electronegative elements.
There are some elements also which exhibit the properties of both metals and non-metals. These are called metalloids. In the modern periodic table, a zig-zag line separates metals from non-metals. The borderline elements — boron, Silicon, germanium, arsenic, antimony, tellurium and polonium are intermediate in properties so they are called metalloids or semi-metals.
Variation Along a Period and a Group
As the effective nuclear charge acting on the valence shell electrons increases across a period, the tendency to lose electrons will decrease. Down, the group, the effective nuclear charge experienced by valence electrons decreases because the outermost electrons are farther away from the nucleus.
Hence, metallic character decreases across a period and increases down a group Non-metallic character. However increases across a period and decreases down a group.
Nature of Oxides
Oxides of the metals are of basic nature while those of non-metals are acidic. This means that along a period, the basic character of the oxides of the elements decreases while their acidic character increases.
On going down in a group of the periodic table, the order is reverse i.e., basic durum of oxides increases and acidic character of oxides decreases.
Electro Negativity
It may be defined as the relative electron attracting tendency of an atom for a shared electron pair in a covalent bond with other atom.
Variation Along a Period and a Group
The electro negativity of the elements increases along a period since the non-metallic character increases. Similarly, it decreases down the group since the non-metallic character decreases.

 Kaysons Publication
Kaysons Publication
