Carbon and Its Compounds
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Chapter:- 4
Carbon and its Compounds
Carbon and Its Compounds
Carbon is considered as the third most important element, after oxygen and hydrogen, for the existence of life on the earth.
The Covalent Bond
![]()
Thus, there are 4 electrons in its outermost shell and its octet can be completed by the following two ways
- It could lose 4 electrons and form C4+ cation. But a massive amount of energy is required to remove 4 electrons leaving behind a carbon cation with 6 protons in its nucleus holding on just two electrons together.
- It could gain 4 electrons and can form C4- anion. But for a nucleus having 6 protons, it would be difficult to hold on 10 electrons, i.e. 4 extra electrons.
In order to overcome this problem, carbon shares its valence electrons with other atoms of itself or atoms of other elements.
- This type of bonding is called covalent bonding.
- The bonds which are formed by the sharing of an electron pair between two same or different atoms are known as covalent bonds.
- The number of electrons shared show the covalency of that atom.
Formation of Hydrogen Molecule
Atomic number of H = 1
![]()
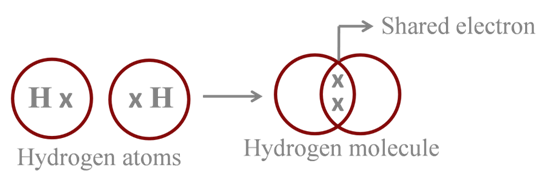
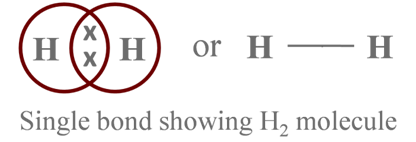
The shared pair of electrons constitutes a single bond between the two H-atoms
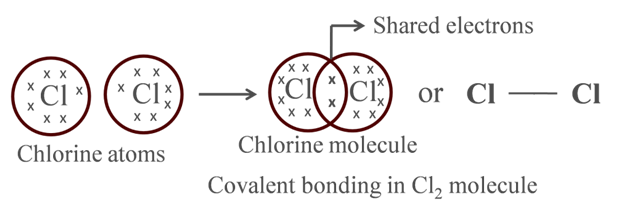
Formation of Chlorine Molecule
Atomic number of Cl = 17
![]()
It has 7 electrons in its outermost shell and thus requires 1 more electron to fulfill its outermost shell.
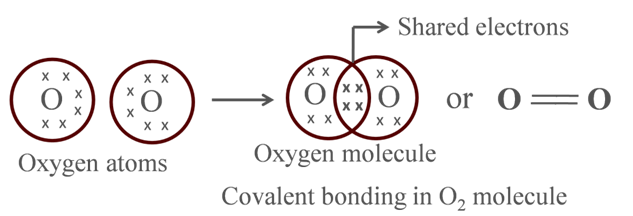
Formation of Oxygen Molecule
Atomic number of O = 8
![]()
It requires 2 electrons to fulfill its octet for attaining noble gas configuration.
Formation of Nitrogen Molecule
Atomic number of N = 7
![]()
It needs 3 more electrons to attain noble gas configuration.
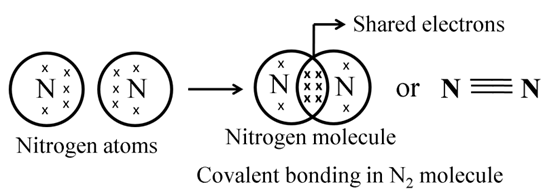
Formation of Methane (CH4)
In the formation of a methane molecule one carbon atom shares its 4 electrons with four different hydrogen atoms
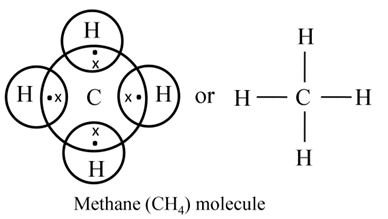
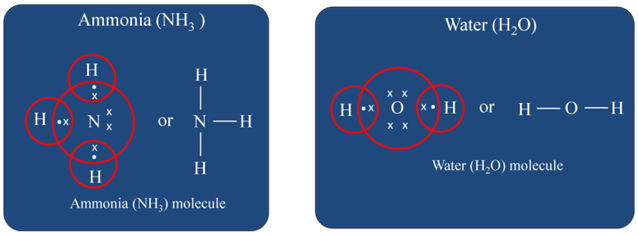
Formation of Ammonia and Water Molecule
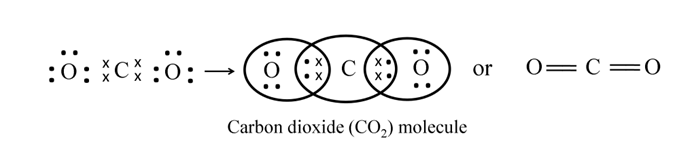
Formation of Carbon Dioxide (CO2)
Atomic number of C = 6
Formation of Sulphur Molecule (S8)
Atomic number of sulphur = 16
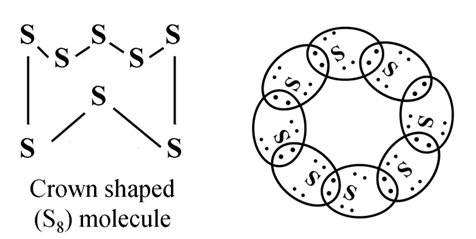
Properties of Covalent Compounds
Covalently bonded molecules are called covalent compounds.
- Their constituents are molecules, not ions.
- They have strong bonds within the molecule but intermolecular forces are weak, which is responsible for low melting and boiling points of these compounds (except graphite and diamond).
- In these compounds, electrons are only shared and no charged particle is formed. Therefore, these compounds are bad conductors of electricity due to the absence of free electrons or ions. However, graphite is an exception of it, which is a good conductor of electricity.
- These compounds are generally insoluble in water but some of them are capable to form H bond which are soluble in water.
Difference between Covalent and Ionic Compounds
- Covalent compounds have lower intermolecular force in comparison with ionic compounds.
- Covalent compounds have comparatively lower boiling and melting points than the ionic compounds.
- Covalent compounds are poor conductors of electricity while ionic compounds are good conductors of electricity in their aqueous solution or in molten state.
- Covalent compounds are formed by the sharing of electrons where as ionic compounds are formed by the lose or gain of electrons.
Allotropes of Carbon and Their Properties
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Allotropes of Carbon and Their Properties
Allotropy is the property by virtue of which an element exists in more than one form and each form has different physical properties but identical chemical properties. These different forms are called allotropes.
Carbon exists in two differents allotropic forms
- Crystalline form
e.g. Diamond, graphite and fullerene.
- Non-crystalline form or amorphous form
e.g. Coal, larrrginriark and charcoal.
Diamond General Properties
- It is a colourless transparent substance with extraordinary brilliance due to its high refractive index.
- It is quite heavy.
- It is extremely hard (hardest natural substance known).
- It does not conduct electricity (because of the absence of free electrons).
- It has high thermal conductivity and high melting point.
- It burns on strong heating to form carbon dioxide.
Structure
It is a giant molecule of carbon atoms in which each carbon atom is linked to four other carbon atoms by strong covalent bonds forming a rigid three-dimensional network structure, which is responsible for its hardness.
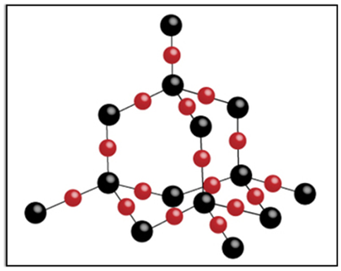
Uses
- Due to its hardness it is used in knives for cutting marble, granite and glass.
- It is used for the purpose of ornaments studded as precious stones.
- It is used as an abrasive and for polishing hard surface.
- Dies made from diamond are used for drawing wires from the metals.
Graphite General Properties
- It is a grayish black opaque substance.
- It is lighter than diamond, feels soft and slippery to touch.
- Is a good conductor of electricity (due to the presence of free electrons) but bad conductor of heat?
- On strong heating, it burns to give carbon dioxide.
Structure
A graphite crystal consists of layers of carbon atoms or sheets of carbon atoms. Each carbon atom in a graphite layer is joined to three other carbon atoms by strong covalent bonds to form flat hexagonal rings. The various layers of carbon atoms.
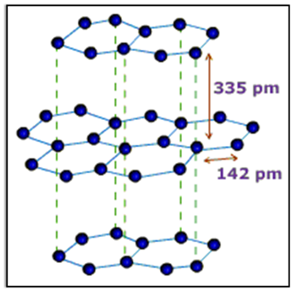
Uses
- Is used as a powdered lubricant for the parts of machinery.
- It is used for making electrodes of cells.
- It is used for making lead for pencils as it can mark paper black. It is therefore called black lead or plumbago.
- It can withstand high temperature so it is used for making crucibles to melt substances with high melting points and tiles on the nose cone of space shuttle.
Fullerenes
These are recently discovered allotropic forms of carbon which were prepared for the first time by H W Kroto, Smalley and Robert Curt by the action of laser beam on the vapours of graphite.
General Properties
- These are dark solids at room temperature.
- These are neither too hard nor too soft.
- These are the purest allotropic forms of carbon because of the absence of free valencies or surface bonds.
- On burning, these produce only carbon dioxide gas. Other properties of fullerene are still being investigated.
Structure
Fullerene (C60) is a football shaped spherical molecule in which 60 C atoms are arranged in interlocking hexagonal and pentagonal rings of carbon atoms.
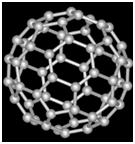
Uses
- In pure form, these behave as insulators. However, these can be converted into semiconductors under suitable conditions.
- C60O, a molecule formed when C60 traps O atoms, is used in cancer as well as AIDS therapy.
- In small amounts, these are used to catalyses the photochemical refining in industry.
Versatile Nature of Carbon
The estimated number of carbon compounds known today is about three million.
The main reasons for this huge number of carbon compounds are
Catenation
The property of self linking of elements mainly carbon atoms through covalent bonds to form long, straight or branched chains and rings of different sizes is called catenation.
Tetravalency of Carbon
Carbon has four electrons in the outermost shell. Hence, its valency is four i.e. it is capable of bonding or pairing with four other carbon atoms or with the atoms of some other monovalent elements like hydrogen, halogen (chlorine, bromine) etc.
Tendency to form Multiple bonds
Due to its small size carbon has a strong tendency to form multiple bonds (double and triple bonds) by sharing more than one electron pair with its own atoms or with the atoms of elements like oxygen, nitrogen, sulphur etc.
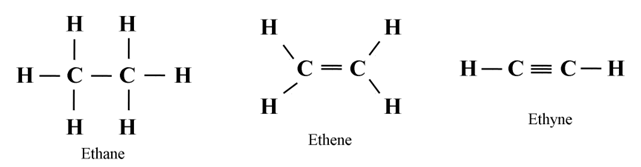
Organic Compounds
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Organic Compounds
The compounds of carbon except its oxides, carbonates and hydrogen carbonate salts, are known as organic compounds. These compounds were initially extracted from natural substances.
Hydrocarbons
The organic compounds containing only carbon and hydrogen are called hydrocarbons.
The hydrocarbons can be classified as
- Saturated hydrocarbons
- Unsaturated hydrocarbons
Saturated Hydrocarbons
The hydrocarbons in which all the carbon atoms are connected by only single bonds are called saturated hydrocarbons or alkanes.
The general formula of these compounds is CnH2n + 2
Unsaturated Hydrocarbons
Those carbon compounds in which at least one double or triple bond is present are called unsaturated compounds.
Unsaturated compounds are further divided into following two classes
- Alkenes (CnH2n)
- Alkynes (CnH2n – 2)
Alkenes
Those carbon compounds which have at least one double bond along with single bonds are called alkenes.
General formula of these compounds is CnH2n.
Alkynes
Those unsaturated hydrocarbons which have one or more triple bonds along with the single bonds are called alkynes.
General formula of these compounds is CnH2n – 2.
How to Draw the Structure of Saturated and Unsaturated Compound?
To draw the structure of carbon compound
Step I:- First connect all the carbon atoms by a single bond.
Step II:- After that satisfy the tetravalency of carbon with available hydrogen atom.
Step III:- If number of available H atoms are less than what is required, satisfy the remaining valency by using double or triple bond in between C atoms.
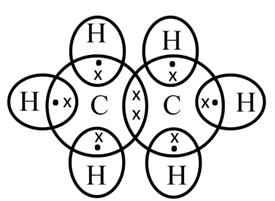
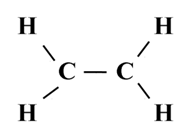
Structure of Ethane (C2H6)
![]()
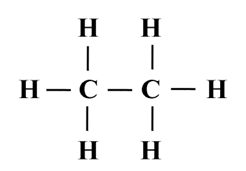
Electron dot structure of ethane (C2H6)
Structure of Propane (C3H8)
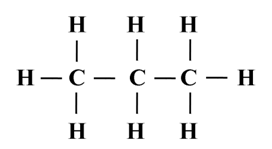
Electron dot structure of propane (C3H8)
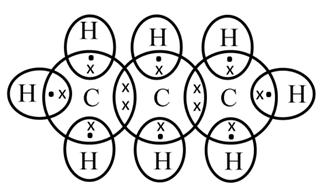
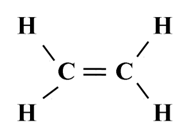
Structure of Ethene (C2H4)
![]()
To satisfy it, a double bond is used between the two carbon atoms.
Electron dot structure of ethane
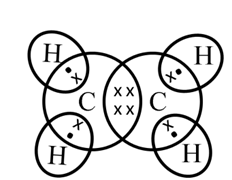
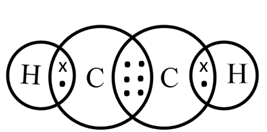
Structure of Ethyne (C2H2)
C ─ C
H ─ C ≡ C ─ H
In ethyne, the two carbon atoms share three pairs of electrons among themselves to form a carbon-carbon triple bond.
Electron dot structure of ethyne (C2H2)
How to Draw Structure of Cyclic Compound?
Some carbon structure also exist in cyclic or ring structure.
To draw the structure of cyclic or ring compound
Step I:- First connect the available carbon atoms by a single bond in the cyclic form.
Step II:- Try to satisfy the tetra valency of each carbon with the available hydrogen atoms.
Step III:- Now check the valency of each carbon. If it is found unsatisfied, use double or triple bond to satisfy it.
For example
Isomerism
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Isomerism
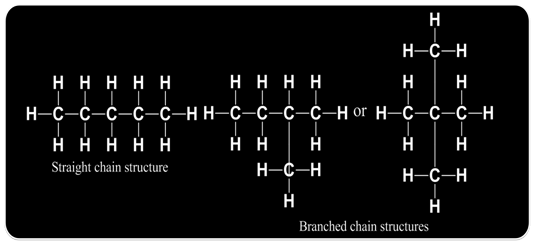
Carbon compounds or organic compounds with same molecular formula can show different structures and hence, different properties. This phenomenon is called isomerism and compounds are called isomers.
For example
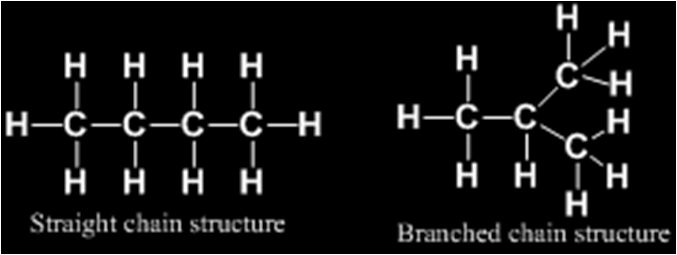
Such pair of isomers is called chain isomers and the isomerism is called chain isomerism.
Molecular formula C5H12
Functional Groups
Functional groups may be defined as an 'atom' or a ‘group of atoms’ which makes a carbon compound reactive and decide its properties regardless of the length and nature of carbon chain.
Homologous Series
A series of similarly constituted compounds in which the members present have the same functional group and similar chemical Properties and any two successive members in a particular series differ in their molecular formula by —CH2— unit, is called a homologous series.
Characteristics of a Homologous Series
- All the members of a homologous series can be represented by the same general formulae.
- Any two adjacent homologues differ by 1 carbon atom and 2 hydrogen atoms in their molecular formulae.
- All the Compounds of a homologous series show similar chemical properties.
- With increase in the molecular mass the gradual change in the physical properties occurs.
- The 14 u is the difference in the molecular masses of any two adjacent homologues:
For example,
CH4, C2H6, C3H8, C4H10 — these all compounds differ by a — CH2 — unit.
Nomenclature of Carbon Compounds
Organic compounds have generally two types of names. These are IUPAC names and common names. The IUPAC names have been adopted by the International Union of Pure and Applied Chemistry. And are based on certain rules. The common names, also known as trivial names, have no proper system for naming.
In general, the IUPAC names of organic compounds are based on the name of basic carbon chain modified by a prefix (phrase before) or suffix (phrase after) showing the name of the functional group.
Writing IUPAC Name of a Compound
Following steps are used to write the name of an organic compound
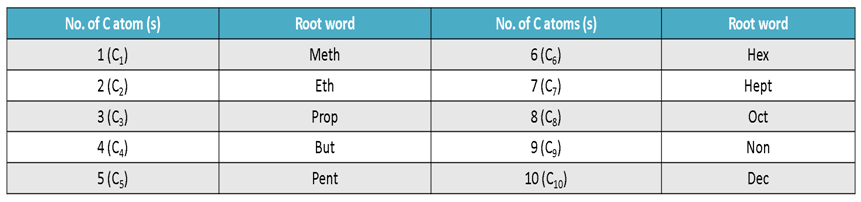
Step I:- Count the number of carbon atoms in the given compound and write the root word for it.
Step II:- If the compounds is saturated, add suffix ‘ane’ to the root word, but if is unsaturated, add suffix ‘ene’ and ‘yne’ for double and triple bonds respectively.
Step III:- If functional group is present in the compounds, it is indicated by adding its suffix.
Root Words for Carbon Atoms
Combustion AND Oxidation
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Combustion And Oxidation
Combustion
All the carbon compounds burn in oxygen and yield carbon dioxide and water vapours. Heat and light are also released during this process. This reaction is called combustion.
For Example
C + O2 → CO2 + Heat + Light
CH4 + 2O2 → CO2 + 2H2O + Heat + Light
2CH3CH2OH + 6O2 → 4CO2 + 6H2O + Heat + Light
Saturated hydrocarbons give a clean flame due to their complete combustion whereas, unsaturated hydrocarbons give a yellow flame with lots of black smoke as they do not undergo complete combustion.
Oxidation
It is the process of intake of oxygen and removal of hydrogen. Reagents used for this purpose are alkaline KMnO4 + heat or acidified K2Cr2O7 + heat.
For Example
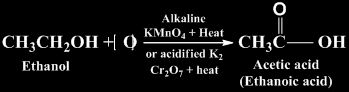
Substances that are capable of providing oxygen to other substances are called oxidising agents.
![]()
Addition Reactions
Addition of hydrogen (hydrogenation) in the presence of catalysts like palladium or nickel, to unsaturated hydrocarbons yields saturated hydrocarbons.
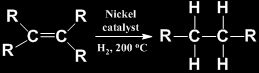
Hydrogenation of vegetable oil in the presence of nickel catalyst gives ghee. This process is called hardening of oils.
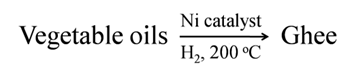
Substitution Reactions
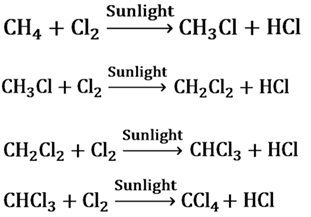
The reactions in which a reagent substitutes an atom or a group of atoms from the reactant are called substitution reaction.
When chlorine is added to hydrocarbons at a rapid rate in the presence of sunlight, Cl replaces H atom one by one.
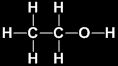
Some Important Carbon Compounds Ethanol
Its common name is ethyl alcohol and formula is C2H5OH or CH3CH2OH
Preparation
Alcohol (ethanol) is obtained by the fermentation of molasses which are obtained from sugarcane juice.
![]()
Physical Properties
Physical properties of ethanol are
- It is a liquid at room temperature. Its melting point is 156 K and boiling point is 351 K.
- It is Soluble in water due to its ability to form H- bonds with water molecules.
Chemical Properties
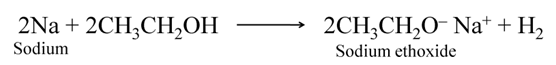
Reaction with Sodium
Ethyl alcohol reacts with sodium metal leading to the evolution of hydrogen gas along with the formation of sodium ethoxide.
Dehydration
Removal of water molecules from a compound is known as dehydration reaction.
For example
When ethanol is heated at 443K with excess conc. H2SO4, the water molecules get removed from it and ethane is obtained.

Uses
Uses of ethanol are
- It is used as an active ingredient in all alcoholic drinks.
- It is useful in medicines like tincture of iodine, cough syrups and may other tonics.
- Alcohol is used as an additive in petrol, since it is a clear fuel and gives rise to only CO2 and H2O when burnt in sufficient air.
- It is used as hypnotic (induces sleep).
- It is used for the preparation of chloroform, iodoform, ethanoic acid, ethanal, etc.
Denatured Alcohol
In order to stop the misuse of ethanol, it is made unfit for drinking by adding poisonous substances like methanol, coppet sulphate, pyridine, etc and coloured substances like dyes. Such alcohol is called. Denatured alcohol.
Ethanoic Acid or Acetic Acid
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Ethanoic Acid or Acetic Acid
It is commonly known as acetic acid. Its formula is CH3COOH. 5-8% solution of ethanoic acid in water is known as vinegar.
Physical Properties
- Its melting point is 290K.
- During winters it often freezes and form ice like flakes, so it is also called glacial acetic acid.
Chemical Reactions
Acidity It is a weaker acid than HCl but stronger than alcohol. It evolves hydrogen when reacts with sodium metal or sodium hydroxide, which shows its acidic nature.
2CH3COOH + 2Na → 2CH3COONa + H2 ↑
CH3COOH + NaOH → CH3COONa + H2O ↑
Esterification When ethanol (an alcohol) reacts with acetic acid (a carboxylic acid), a fruity (sweet) smelling liquid called ester is obtained. This reaction is called esterification.
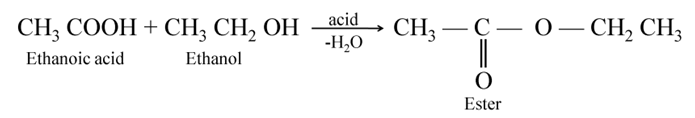
Saponification
The ester gets converted back into alcohol and sodium salt of acid when treated with alkali like sodium hydroxide. This reaction is called saponification, as it is used for the preparation of soap.
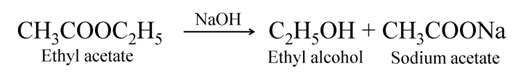
Reaction with carbons and hydrogen carbonates in the reaction of acetic acid with carbonates or hydrogen carbonates, carbon dioxide gas is obtained. It is an example of acid-base reaction.
![]()
![]()
Uses of Acetic Acid
- It is used for making vinegar.
- It is widely used as a preservative in pickles.
- It is used for the synthesis of other compounds like esters.
Soaps and Detergents
Soaps are sodium or potassium salts of long chain carboxylic acids and have general formula RCOONa.
Detergents are usually ammonium or sulphonate salts of long chain carboxylic acids.
Manufacture of Soaps and Detergents
Soaps are made from animal fat or vegetable oils by heating it with sodium hydroxide.
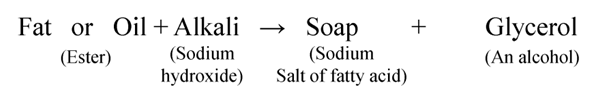
This process of preparation of soap by the hydrolysis of fats and oil with alkalies is called saponification.
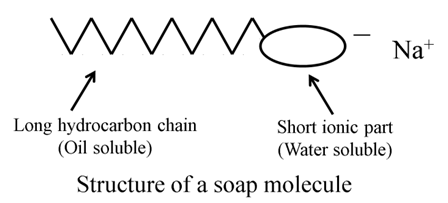
Structure of a Soap Molecule
A soap molecule is made up of two parts — a long hydrocarbon part and a short ionic part containing — COONa group. The long hydrocarbon part is hydrophobic and therefore insoluble in water but soluble in oil. The ionic portion of the soap molecule is hydrophilic so, soluble in water and insoluble in oil.
Cleansing Action of Soaps (Micelle Formation)
Some molecules have different properties at their two ends. Its one end is hydrophilic (soluble in water) and other is hydrophobic (soluble in fats or oils).
Inside water these molecules show a unique orientation that keeps the hydrocarbon portion out of the water. This is done by forming clusters of molecules in which the hydrophobic tails are in the interior of the cluster and on the surface of cluster ionic ends are present. This formation is called micelle formation.
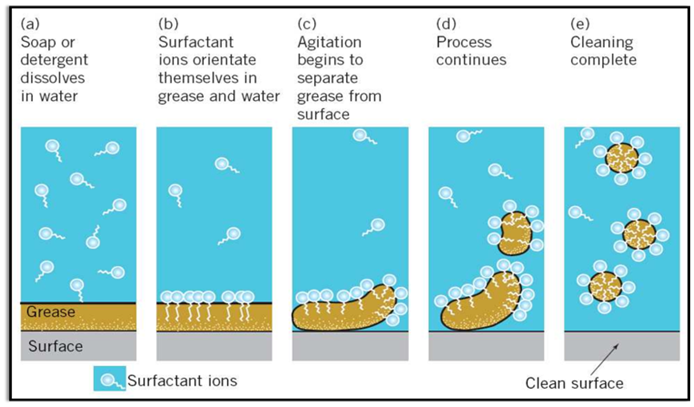
Limitations of Soap
Soap is not suitable for washing clothes with hard water. Because hard water contains calcium and magnesium salts. Soap forms scum with hard water as it reacts with calcium and magnesium ions present in the hard water.
Detergents are used as cleansing agents in case of hard water become charged ends of detergents do not form insoluble precipitates with magnesium and calcium ions present in hard water. Hence, they are effective in hard water.
Detergents are useful in making shampoos and products for cleaning clothes but their main disadvantage is that these are generally non-biodegradable.
Advantages of Detergents Over soaps
Synthetic detergents do not require vegetable oil or fats for their preparation, hence they help in saving oils and fats for human consumption.
Synthetic detergents can be used with hard water while soaps cannot be used with hard water.

 Kaysons Publication
Kaysons Publication
