Metals
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Chapter:- 3
Metals and Non-metals
Introduction
About 118 chemical elements are known at present. On the basis of their properties, all the elements can be divided into two main groups i.e., metals and non-metals. Apart from metals and non-metals, some elements show properties of both metals and non-metals. These are called metalloids.
Both metals and non-metals are very important in our everyday life. We also use a large number of compounds of metals and non-metals.
Metals
Elements that are electropositive in nature are called metals. They have a tendency to lose electrons and form positive ions. For example, copper, iron, aluminium, sodium, etc.

Physical Properties of Metals
- Malleability It is the property of metal due to which it can be beaten into thin sheets.
- Ductility It is the property due to which a metal can be drawn into wires.
- Hardness Most of the metals are hard. But some alkali metals like sodium and potassium are so soft that they can be cut easily with knife.
- Metallic Lustre Metals in their pure state have bright shining surfaces.
- Electrical Conductivity Most of the metals are good conductors of electricity.
- Thermal Conductivity Generally metals are good conductors of heat, except lead and mercury.
- Melting and Boiling Points Metals generally have high melting and boiling point, except mercury, gallium and alkali metals.
- Sonority When metals are struck with a hard substance, they produce sound.
Chemical Properties of Metals
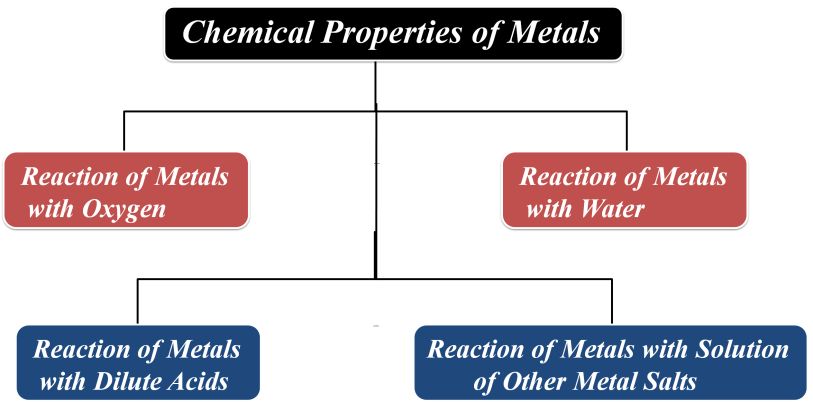
Most of the chemical properties of metals are due to their electropositive nature. It means that a metal atom lose electrons to form cations.
Reaction of Metals with Oxygen
Burning in Air (Formation of oxides)
Almost all the metals react with oxygen (or air) to form metal oxides.
![]()
For example, when copper is heated in air, it combines with oxygen to form copper (II) oxide, a black oxide.
![]()
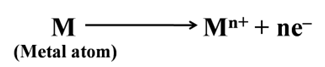
Similarly, sodium forms sodium oxide
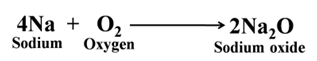
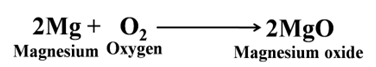
Magnesium forms magnesium oxide
Aluminium forms aluminium oxide
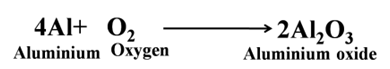
Nature of Metallic Oxides
(Formation of hydroxides)
Most of the metallic oxides are basic in nature. When dissolved in water, they produce alkali. For Example:-
Similarly, sodium oxide forms sodium hydroxide
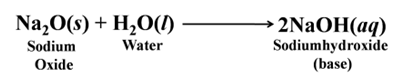
Note:- Alkalis are the bases that dissolve in water.
Exception
Some metal oxides show both types of behavior, i.e., acidic and basic. These oxides are called amphoteric oxides. e.g., zinc oxide, aluminium oxide, etc. For Example:-
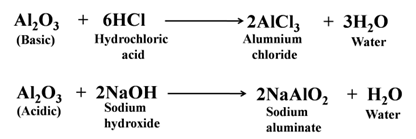
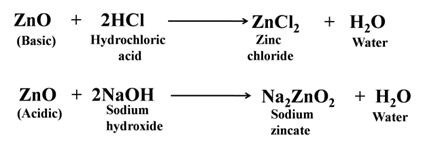
Order of Reactivity with Oxygen
Different metals react with oxygen at different rates. For example, sodium (Na) and potassium (K) catch fire, when placed in the open. Hence, these are most reactive metals. To prevent accidental fires, these metals are kept immersed in kerosene oil. Magnesium (Mg) burns in air only by heating. Zinc (Zn) burns only on strong heating while iron does not burn in the form of rod or block but burns in the form of filings. Copper (Cu) does not burn on heating but blister copper burns.
Oxide silver does not react with oxygen. Hence, the order of reactivity of these metals with oxygen is Na > Mg > Zn > Fe > Cu > Ag.
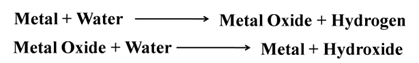
Reaction of Metals with Water
Metals react with water and produce a metal oxide and hydrogen gas. Metals oxides that are soluble in water dissolve into further to form metal hydroxides but all metals do not react with water.
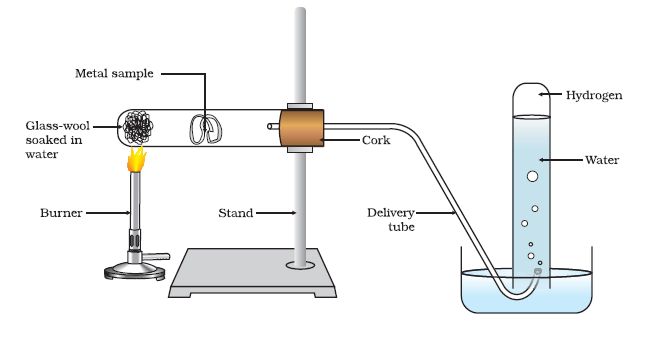
Metals like potassium and sodium react violently with cold water. In case of Na and K, the reaction is very violent and exothermic.
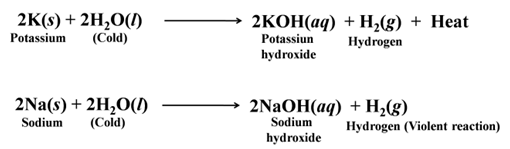
The, heat evolved is sufficient for hydrogen to catch fire. That’s why Na and K catch fire when kept in water.
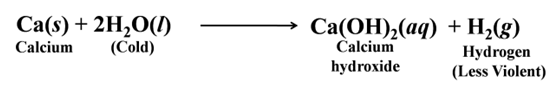
The reaction of calcium with water is less violent. The heat evolved is not sufficient for the hydrogen to catch fire.
Magnesium does not react with cold water. It reacts with hot water and forms magnesium hydroxide and hydrogen.
It also starts floating due to the bubbles of hydrogen gas sticking to its surface.
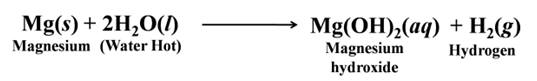
Metals like aluminium, iron and zinc do not react either with cold or hot water. They react with steam and form the metal oxide and hydrogen.
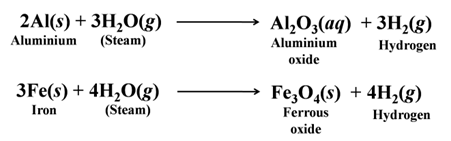
Lead, copper, silver and gold do not react with water at all. Thus, the reactivity order of metals towards water is
K > Na > Ca > Mg > Al > Fe > Pb > Cu > Ag > Au.
Reaction of Metals with Dilute Acids
- Reaction of Metals with Dilute HCl and Dilute H2SO4.
Except a few less reactive metals (Cu, Hg, Ag, Au, Pt, etc) all metals react with dilute sulphuric acid and hydrochloric acid to produce salt and hydrogen gas. For Example:-
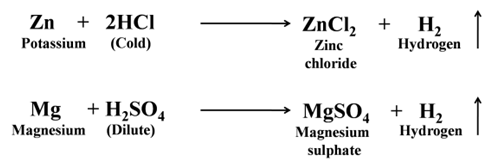
Reaction of Metals with Dilute HNO3 Except magnesium and manganese, metals do not react with dilute nitric acid. This is due to the oxidising nature of nitric acid.
Normal Case:-
![]()
![]()
Exceptional Case (for Mn and Mg only)
Aqua Regia
It is a freshly prepared mixture of concentrated hydrochloric acid and concentrated nitric acid in the ratio of 3 : 1. It can dissolve gold, even though neither of these acids can do so alone.
Aqua regia is a highly corrosive, fuming liquid; It is one of the few reagents that is able to dissolve gold and platinum.
Reaction of Metals with Solution of Other Metal Salts
A reactive metal can displace a comparatively less reactive metal from their compounds in aqueous solution or in molten state.
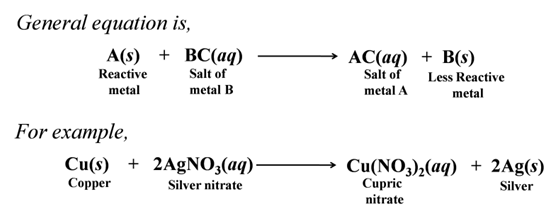
Reactivity Series or Activity Series of Metals
The arrangement of metals in the order of their decreasing reactivities is called reactivity series of metals.
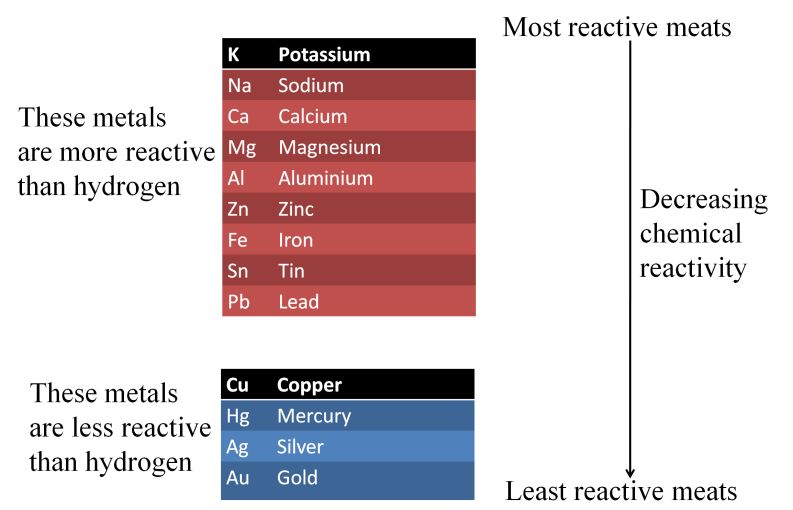
Importance of Reactivity Series
The following two points state importance of reactivity series
- In reactivity series, metals that are placed above hydrogen, can displace hydrogen from dilute acids (HCI and H2SO4).
- Metal placed above another metal in this series can displace it from the solutions of its salt, as the metal towards top is more reactive than a metal placed below it.
Non-Metals
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Non-Metals
Elements that are electronegative in nature are called non-metals. It means non-metals gain electrons to form negative ions.
For Example, iodine, sulphur, hydrogen, etc.

Physical Properties of Non-Metals
- Brittleness Non-metals are neither malleable nor ductile but they are brittle in nature.
- Physical State Most of the non-metals are soft (if solid). Only diamond, a form of carbon is the hardest known substance. Other non-metals are gases except bromine which is a liquid.
- Metallic Lustre the non-metals do not have lustre. However, diamond, iodine and graphite, have lustre.
- Electrical and Thermal Conductivity Non-metals are generally poor conductors of heat and electricity except graphite.
- Melting and Boiling Points Generally, non-metals have low melting and boiling points. But non-metals that are solids have comparatively higher boiling points. (e.g., B. Si, C, etc.)
Chemical Properties of Non-Metals
- Reaction with Oxygen Non-metals react with oxygen to form oxides. These oxides are generally acidic. Only some of the non-metallic oxides are neutral.
Acidic oxides are CO2, SO2, P2O5, etc. For Example:-
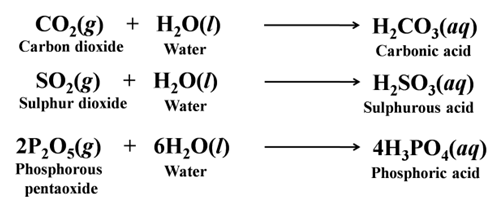
Neutral oxides are CO, H2O, N2O, etc.
Reaction with Water Non-metals do not react with water or steam to evolve hydrogen gas. This is because non-metals cannot give electrons to hydrogen in water therefore, hydrogen gas cannot be released.
Reaction with Acids Non-metals do not react with acids to release hydrogen gas. Reason is that non-metals cannot loose electrons and give it to hydrogen ions of acids so, that the gas is released.
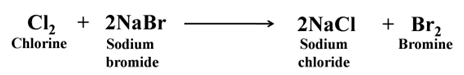
Displacement Reaction Non-metals also show displacement reaction like metals. For Example:-
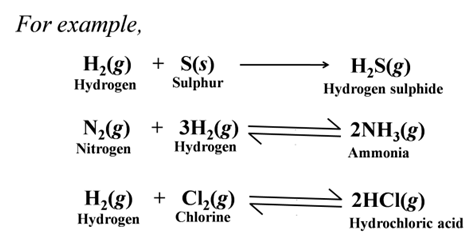
Formation of Covalent Compounds Non-metals form covalent compounds with other non-metals like hydrides, chlorides, etc.
Exceptions Among Metals and Non-metals
- All metals (except mercury, a liquid metal) exist in solid state at normal room temperature.
- Gallium and cesium, (metals) have very low melting point. When kept on the palm, they start melting.
- Iodine, diamond and graphite are lustrous although they are non-metals.
- Diamond has a very high melting point and is very hard although it is a non-metal.
- Alkali metals have Low densities and low melting points. They are too soft to be cut with a knife.
- Graphite is a good conductor of electricity although it is non-metal.
Reaction of Metals and Non-Metals
Formation of Ionic Compounds
We know that noble gases are unreactive and the reason for their unreactive nature is the presence of 8 electrons in their outermost shell (octet) with an exception of helium (which has duplet i.e., two electrons in the outermost or the only shell).
The elements try to get 8 electrons in their outermost shell as it is the state of maximum stability or
minimum energy (by gaining or losing electrons).
To recall them, see the following table:-

Formation of Sodium Chloride
The two oppositely charged ions (sodium and chloride ions in the below case) bind together with the help of strong electrostatic forces of attraction which is termed as ionic bond or electrovalent bond and the compounds having it are called ionic or electrovalent compounds.
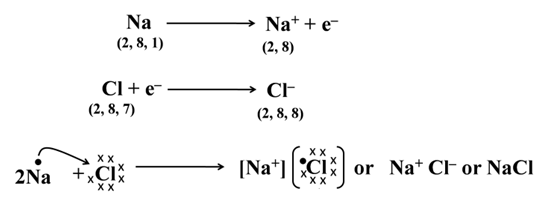
Thus, it is also clear that ionic compounds (like sodium chloride) do not exist as discrete molecules but indeed they are the aggregates of oppositely charged particles.
Formation of Calcium Chloride
Formation of ionic bond, can further be understood by taking an example of calcium chloride (where calcium is a metal and chlorine is a non-metal), in which calcium (Ca) with configuration K, L, M, N have a tendency to lose teo electrons and chlorine with configuration K, L, M have a tendency to gain one electron.
Thus, the two electrons lost by Ca are achieved by two Cl atoms. The Ca atom acquires two positive charges (Ca2+) and each Cl atom acquires a negative charge (Cl–). These oppositely charged ions are held together by electrostatic forces i.e., by ionic bond.
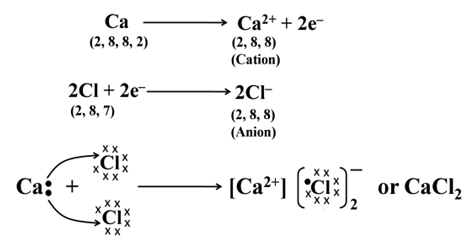
Properties of Ionic Compounds
- Physical Nature Ionic compounds are crystalline solids.
- Melting and Boiling Points These compounds have high melting and boiling points as large amount of energy is required to break strong forces of attraction.
- Solubility These compounds are soluble in water and insoluble in organic solvents like kerosene, benzene, ether, petrol, etc.
- Conduction of Electricity Ionic compounds are good conductors of electricity, but they conduct electricity either in molten state or in their aqueous solution.
Occurrence and Extraction of Metals
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Occurrence and Extraction of Metals
The earth's crust is the major source of metals. Sea water is also a source for some metals. It contains soluble salts like sodium chloride, magnesium chloride, etc.
Minerals The naturally occurring elements or compounds of metal present in the earth’s crust are called minerals.
Ores Those minerals from which a particular metal be extracted profitably are called ores. Each ore is a mineral but each mineral is not an ore.
Gangue the undesirable impurities like soil, sand, etc found in ore are called gangue.
Enrichment of Ores Removal of unwanted material (gangue)
Extraction of Metals
Attainment of pure metal from its compound is called extraction of metals. Some metals are found in earth’s crust in Free State while some are found in the form of their compounds. The metals present at the bottom of the reactivity series are least reactive so they are found in Free State. e.g., Gold, Silver, Platinum, Copper.
The metals at the top of the reactivity series are highly reactive so they are found as free elements. The metals in the middle of the reactivity series (zinc, iron, lead) are moderately reactive, found as oxides, sulphides or carbonates.Copper and silver are found in the combined state in their sulphide or oxide ores.
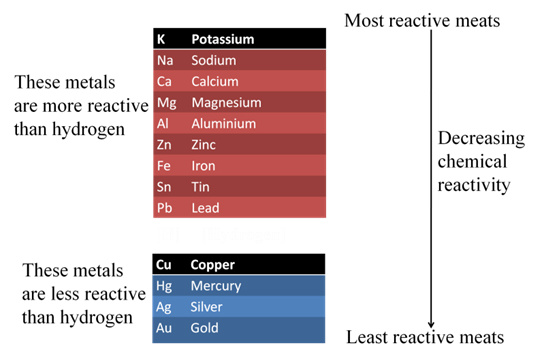
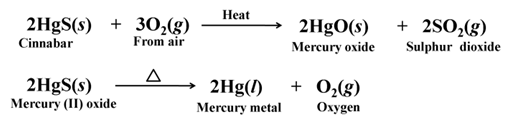
Extracting Metals Low in the Activity Series (Low in activity series)
Cinnabar (HgS) is an ore of mercury, when heated in air, it first changes into its oxide and then into mercury metal.
For example,
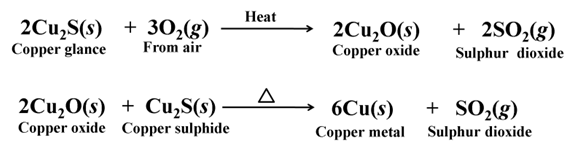
Copper glance (Cu2S) when heated in air, partially oxidised and the oxidised product reacts with the remaining copper glance to give copper metal.
For example,
Extraction of Metals in the Middle of the Activity Series
The metals in the middle of the activity series are usually present as sulphides or carbonates in nature. Sulphides are converted into oxides by roasting and carbonates are converted into oxides by calcination
Roasting

Calcination

Reducation
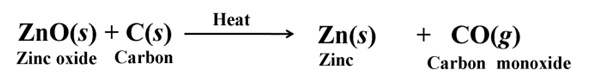
Thermit Reaction
The reaction of iron (III) oxide (Fe2O3) with aluminium is used to join railway tracks or cracked machine parts of heavy machines.
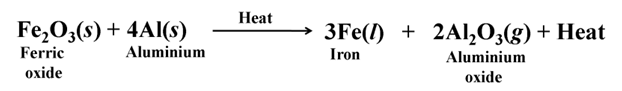
This reaction is known as thermit reaction its highly exothermic.
Extraction of Metals Towards the Top in Activity Series
These metallic compounds cannot be reduced by carbon or any other reducing agent due to their high affinity with oxygen. Therefore, electrolytic reduction is employed for these metals e.g., Na, Mg, Ca, etc.
Electrolytic reduction the salts of these metals as chlorides in molten form are electrolysed. Metal is deposited at the cathode and chlorine is liberated at the anode.
The reactions are (At cathode)
![]()
The reactions are (At anode)
![]()
Refining of Metals
It is the process of purification of the metal obtained after reduction. Various methods for refining are employed, but the most common one is the electrolytic refining.
Electrolytic Refining
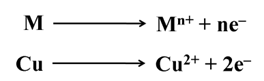
Many metals like Cu, Sn, Ni, Ag, Au, etc are refined electrolytically.
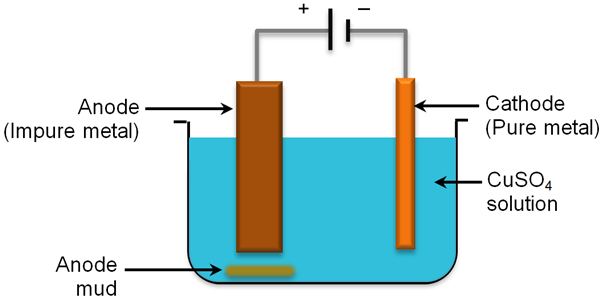
The reactions are (At cathode)

The reactions are (At anode)
Corrosion
It is the process of slow eating away of metals by the reaction of atmospheric air and moisture.For example, rusting of iron, tarnishing of silver, formation of green coating over copper etc.

Prevention of Corrosion
Rusting of iron is prevented by painting, oiling, greasing, galvanising, chrome plating, anodising and by making alloys.
Galvanisation
The process of coating iron and steel objects with a thin layer of zinc is called galvanisation. It is done by dipping the object in molten zinc.

Anodising
It is the process of forming a thick oxide layer on the surface of metal. At ordinary temperature the surfaces of metals like zinc, aluminium, etc are covered with a thin layer of oxide. This oxide layer is protective and prevents the metal from further oxidation. This layer can be made more thick by anodising.
In this process. Clean metal is taken as anode and dilute H2SO4 as electrolyte. When electric current is passed. O2 gas is liberated which reacts with metal to form a layer of oxide.
Alloy
An alloy is a homogeneous mixture of two or more metals or a metal and a non-metal. It is prepared by mixing the metals in molten state and then cooling the mixture. The electrical conductivity and melting point of an alloy is less than that of pure metals.
For example,
- Amalgam If an alloy contains mercury as one of its components, it is called amalgam.
- Alloying of Gold Pure gold is very soft. It is called 24 carat gold. To increase the strength and hardness of gold and to make it suitable for making jewellery, alloy of gold is made with either silver or copper.
- Alloying of Iron Pure iron is very soft and stretches easily when hot. It is mixed with a small amount of carbon (about 0.05%) and it becomes strong.
Alloying
It is the method of improving the properties of a metal by mixing two or more metals

Purpose of Making Alloy
- Alloy do not corrode or corrode to very less extent.
- Alloy is much harder and stronger than the pure metal.
- Alloy has less conductance than pure metal.
- Some alloys have, low melting point and hence used for soldering of metals.
Alloys, their Composition and Uses
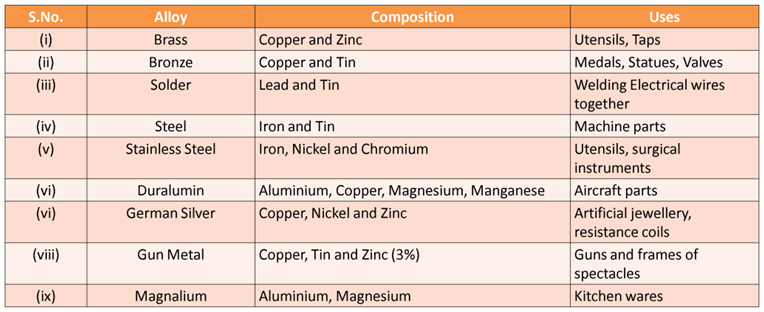
Constituents and Uses of Alloys
Brass (copper and zinc) it is used in making utensils and taps.
Bronze (copper and tin) it is used in making medals, statues, etc.
Solder (lead and tin) it is used to weld electrical wires.

 Kaysons Publication
Kaysons Publication
