Introduction
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Chapter:- 1
CHEMICAL REACTIONS & EQUATIONS
Introduction
We come across a variety of changes around us which may be both physical and chemical. A physical change can be easily reversed, but it is not easy to reverse a chemical change. Some common examples of physical change are evaporation, melting of wax, freezing of water, etc and common examples of chemical change are changing of milk to curd, rusting of iron, digestion of food, etc.
All chemical changes are accompanied by chemical reactions and these are represented with the help of chemical equations. In this chapter, we will study about the various types of chemical reactions and the chemical equations which represent chemical changes.
Chemical Reaction
A chemical reaction is a change in which one or more substance(s) or reactant(s) react to form new substance(s) with entirely different properties. Breaking of the old bonds and formation of the new bonds is responsible for the occurrence of a chemical reaction.
Some examples where chemical reactions take place are
(i):- Digestion of food,
(ii):- Rusting of iron,
(iii):- Photosynthesis,
(iv):- Respiration,
(v):- Burning of fuels
A chemical reaction represents the change of the species taking part in the reaction into new species. The reacting species are known as reactants (The substances that undergo chemical change in the chemical reaction, are the reactants) and the new species formed as a result of the reaction are called products (The new substances formed during reaction, are the products).
A chemical reaction can be identified by either of the following observations:-
(i):- Change in state,
(ii):- Change in colour,
(iii):- Evolution of gas↑,
(iv):- Change in temperature,
(v):- Formation of a precipitate↓.
There are some chemical reactions which can show more than one characteristics.
For example, reaction between lead nitrate and potassium iodide solution shows two characteristics; formation of a precipitate of leads iodide and change in colour (from colourless to yellow).
Similarly, reaction between zinc granules and dilute hydrochloric acid shows two characteristics; evolution of a hydrogen gas and change in temperature (rise in temperature).
Chemical Equation
A chemical equation is the symbolic representation of a chemical reaction. Symbols and formulae of the reactants and products are used for the same.
For example, the reaction of burning of methane gas can be represented by the following equation
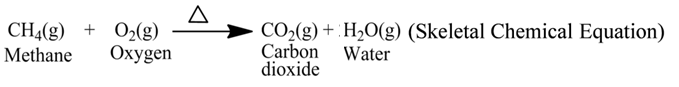
![]()
Making a Chemical Equation More Informative
A chemical equation gives idea about the various substances taking part in a reaction and also about their atoms or molecules included in the reaction. It also tells about the nature of reaction whether it is reversible or not. But following facts remain unexplained with the help of a chemical equation
(i):- Physical states of substances,
(ii):- Reaction conditions,
(iii):- Evolution / absorption of energy
(iv):- Kinetics of the reactions.
Balanced Chemical Equation
A balanced chemical equation is that in which the total number of atoms of each element are equal on both sides of the equation.
Need to Balance a Chemical Equation
According to the law of conservation of mass, 'mass can neither be created nor destroyed during a chemical reaction.'
It means that the total mass of every element present in the products (RHS) of a chemical reaction has to be equal to their total mass present in the reactants (LHS). In other words, the number of atoms of each element remains the same, before and after a chemical reaction. In order to satisfy the law of conservation of mass, a chemical equation should be balanced.
Sodium + Water → Sodium hydroxide + Hydrogen (word equation)
Types of Chemical Reactions
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Types of Chemical Reactions
The chemical reactions are classified into different classes depending upon the types of chemical changes taking place. These reactions are as follows:-
- Combination Reaction
- Decombination Reaction
- Displacement Reaction
- Neutralisation Reaction
- Oxidation and Reduction Reactions
- Exothermic and Endothermic Reactions
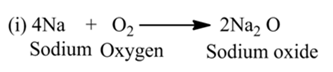
Combination Reaction
A reaction in which two or more reactants react together to form a single product is called a combination reaction.
For example,
(i):- When calcium oxide (quick lime) is dissolved in water, it forms calcium hydroxide (slaked lime).
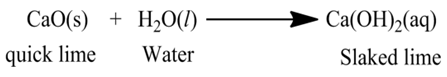
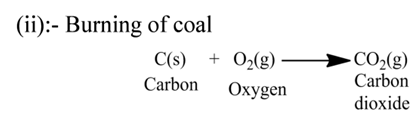
(iii):- Reaction between hydrogen gas and oxygen gas to form water.
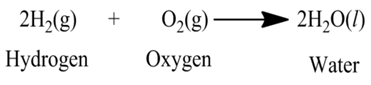
(iv):- Sulphur burns in air to form sulphur dioxide gas.
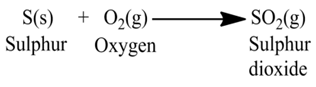
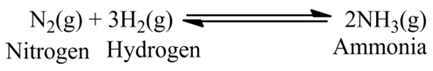
(v):- Nitrogen gas reacts with hydrogen gas to form ammonia gas.
Exothermic and Endothermic Reactions
(a) Exothermic Reactions:-
The reactions which are accompanied by the evolution of heat, are called exothermic reactions or the reactions in which heat is released alongwith the formation of products are called exothermic chemical reactions.
For example,
![]()
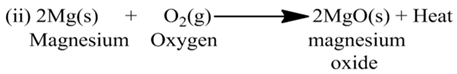
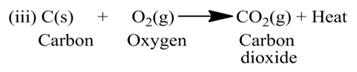
![]()
(b) Endothermic Reactions:-
The reaction, which occur by absorption of heat/energy are called endothermic reactions. It is also called hypothermic reaction. In other words, the energy needed for reaction to occur is less than the total energy released. As a result of this, the extra energy is absorbed in the form of heat.
For example,
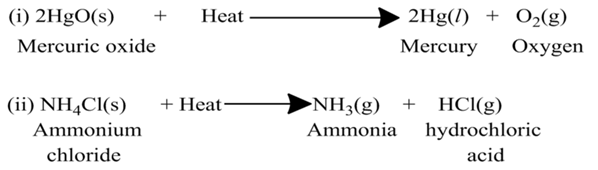

Decomposition Reaction
A reaction, in which a single reactant breaks down to form two or more products, is known as decomposition reaction. On the basis of the form of energy required for the reaction, these reactions are of three types:-
- Thermal Decomposition
- Electroylysis
- Photolysis or Photochemical Decomposition
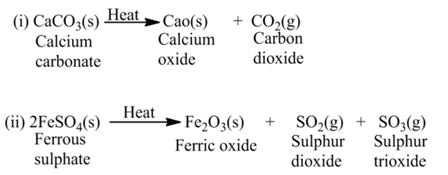
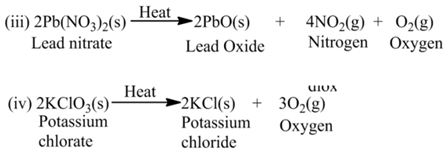
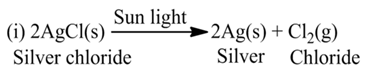
(a)Thermal Decomposition
These reactions use the energy in the form of heat for decomposition of the Reactant. For example,
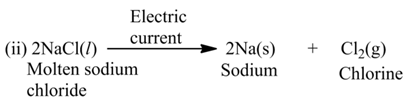
(b) Electrolysis
These reactions involve the use of electrical energy required for the decomposition of the reactant molecules. For example,
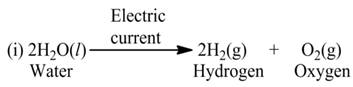
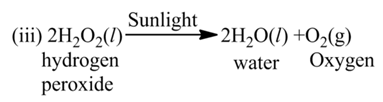
(c) Photolysis or Photochemical Decomposition
These reactions involve the use of light energy for the purpose of decomposition. For example,

Displacement Reaction

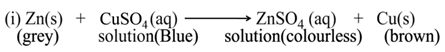
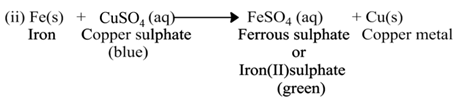
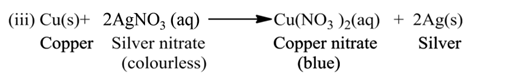
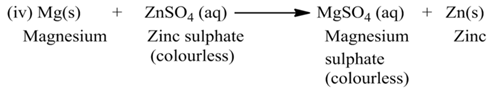
(a) Single Displacement Reaction
A reaction in which a more reactive element displaces a less reactive element from the solution of its compound, is called single displacement or displacement reaction.
For Example:-
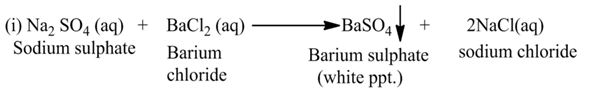
(b) Double Displacement Reaction
The reaction, in which two different ions in the reactant molecules are displaced by each other, is called double displacement reaction. For Example

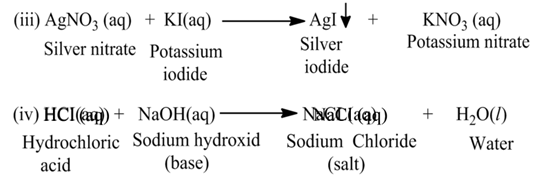
Neutralization Reaction
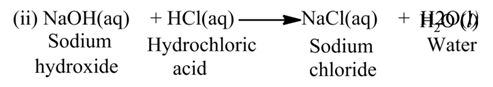
The reaction, in which an acid reacts with a base, is called neutralization reaction.
For Example:-
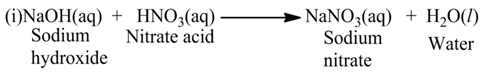
Oxidation and Reduction Reaction
OxidationThe process in which oxygen is added to a substances. OR The process in which hydrogen is removed from a substance.
For Example:-
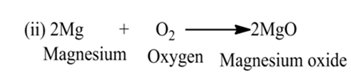
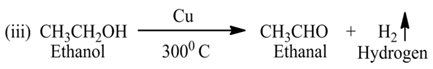
Here, ethanol is oxidized due to the removal of hydrogen.
Reduction
The process in which oxygen is removed from a substance. OR The process in which hydrogen is added to a substance.
For Example:-
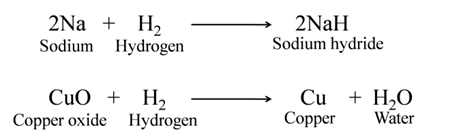
Redox Reactions
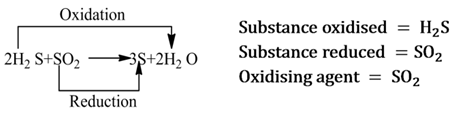
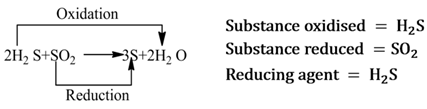
Those reactions in which oxidation and reduction take place simultaneously are called redox reactions.
(i):- In this reaction, the copper (II) oxide is losing oxygen and is being reduced. The hydrogen is gaining oxygen and is being oxidized.
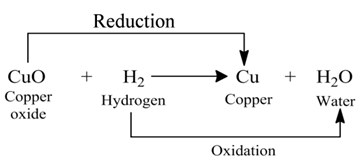
(ii):- In this reaction HCl is oxidised toCl2, whereas MnO2 is reduced to MnCl2.
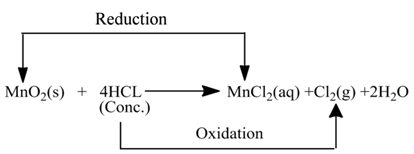
Oxidising Agent
A substance which helps in the oxidation of another substance, is called oxidising agent. It either gives oxygen, removes hydrogen or accepts electrons from the substance to be oxidized.
Note:- It is always to be remembered that oxidising agent itself gets reduced.
Reducing Agent
A substance that helps in the reduction of another substance, is called reducing agent. It either removes oxygen, gives hydrogen or donates electrons to the substance, that is reduced.
Note:- It is always to be remembered that reducing agent itself gets oxidised.
Effects of Oxidation Reaction in Everyday Life
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Effects of Oxidation Reaction in Everyday Life
Corrosion
The phenomenon due to which metals are slowly eaten away by the reaction of air, water and chemicals present in atmosphere, is called corrosion. Some examples of corrosion are:-
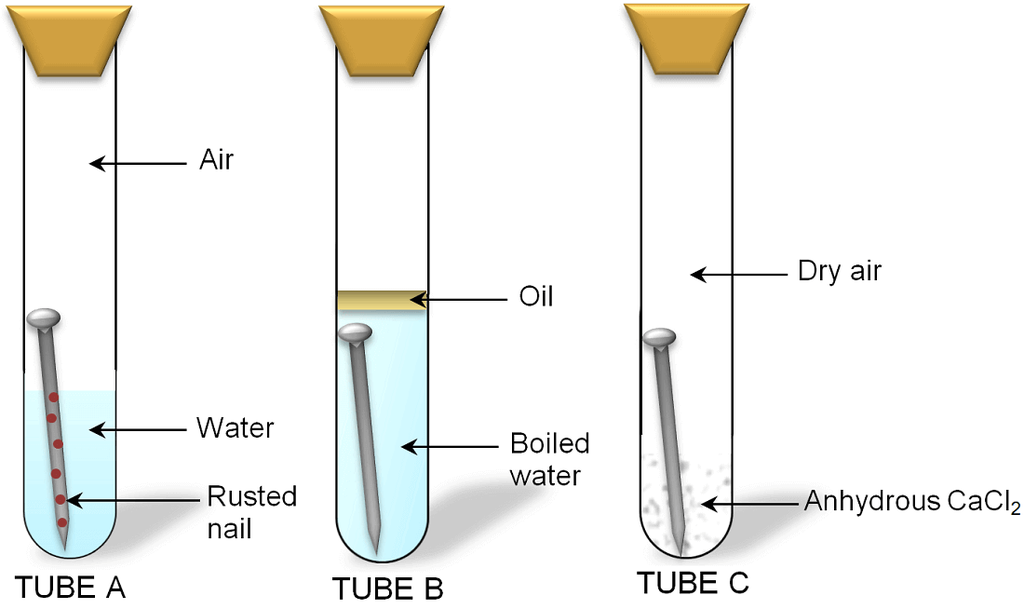
(i) Rusting of Iron Corrosion of iron is called rusting. Iron forms hydrated iron oxide (rust) when kept open in moist air.

(ii) Formation of a Green Layer Over Copper New copper (brown) vessel forms a green layer of basic copper carbonate after some time due to the reaction of air and moisture.
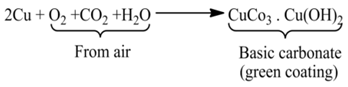
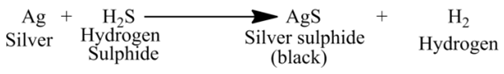
(iii) Tarnishing of Silver although silver is considered a noble metal, yet it reacts with some sulphur compounds as hydrogen sulphide to form black layer of silver sulphide.
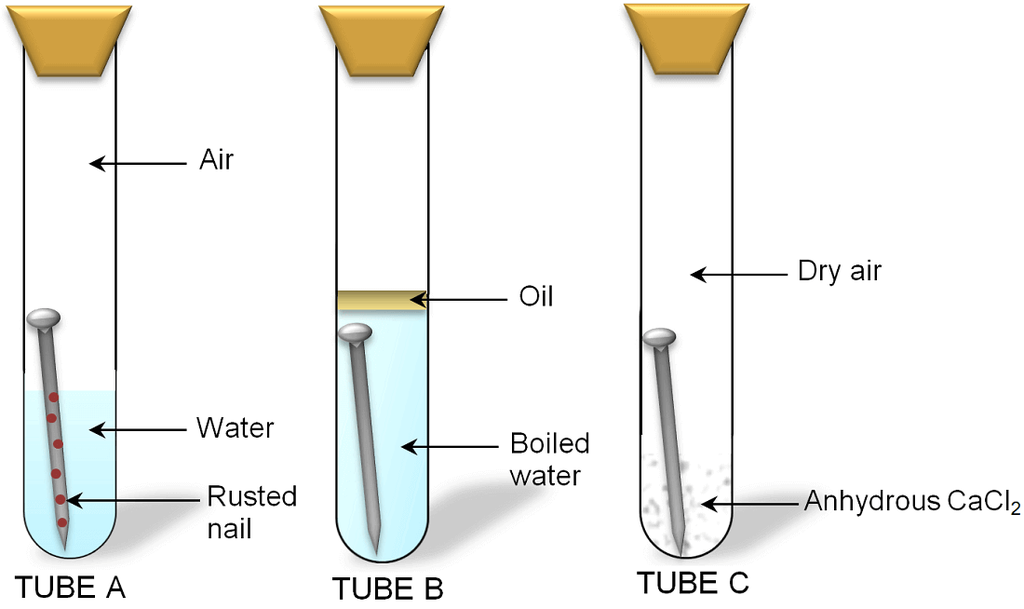
Conditions Necessary For Corrosion
The following two conditions are necessary for corrosion
- Presence of Air
- Presence of Water
Effects of Corrosion
Corrosion causes to damage to car bodies, bridges, iron railing, ships and all objects made of metals, specially those which are made of iron. Corrosion is a wasteful process in most of cases. Every year, a of tones of various metals especially iron get wasted in the country. Hence, it is quite necessary to prevent corrosion.
Exception In case of aluminium, corrosion is not proved to be wasteful. As aluminium is quite reactive metal, the top layer of it forms a thin layer of aluminium oxide which further prevents the corrosion of the metal. It is known as auto-protection mechanism.
Methods to Prevent Corrosion Iron
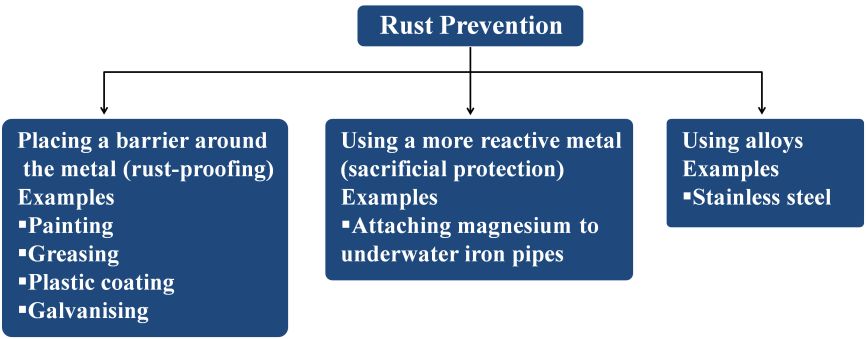
There are three genral methods to prevent corrosion of iron as shown below
Rancitidy
It is the process of slow oxidation of oil and fat present in the food materials resulting in the production of foul odour and taste in them. Conditions under which rancidity take places is when cooked food items are placed for a long time, they become rancid and unsuitable for consumption.
Methods to Prevent Rancidity
The methods to prevent rancidity are:-
- Packing of food materials in air tight containers.
- Refrigeration of cooked food at low temperatures.
- Packing of food items like potato wafers, etc in packets containing nitrogen gas instead of air.
- Avoiding the food materials and cooked food keeping in direct sunlight.
- By adding antioxidants. e.g., BHA (Butylated Hydroxy Anisole) and BHT (Butylated Hydroxy Toluene).

 Kaysons Publication
Kaysons Publication
