Introduction
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Chapter:- 2
Acids, Bases and Salts
Introduction
All the compounds on the basis of their chemical properties can be classified as acids, bases and salts. They have certain definite properties which distinguish one compound class from the other. The sour taste of many fruits and vegetables, (e.g., lemon, orange, amla, etc) is due to various types of acids present in them. The digestive fluids of most humans and animals contain acids. The bitter taste of substance like bitter gourd, cucumber extract, etc is due to bases present in them.
Acids
The word acid comes from the Latin word acidus acere which mean sour. Acids are those chemical substances which have a sour taste and turn blue litmus solution red. Some common fruits such as raw mango, lemon, orange, tamarind, etc are sour in taste.
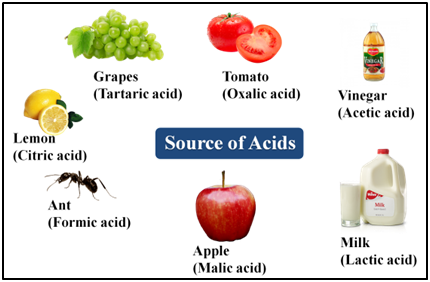
Classification of Acids
- On the Basis of Source
- On the Basis of Ionisaion
- On the Basis of the Amount/Concentration of Acid present in Aqueous Solution
On the Basis of Source
(i):- Inorganic Acids or Mineral Acids These are generally prepared from the minerals present in the earth's crust.
e.g., Hydrochloric acid (HCI), sulphuric acid (H2SO4), nitric acid (HNO3), carbonic acid (H2CO3), sulphurous acid (H2SO3), phosphoric acid (H3PO4), etc.
(ii):- Organic Acids or Edible Acids These are produced by plants or animals.
e.g., acetic acid (Source–vinegar), citric acid (Source–lemon, orange), maleic acid (Source–apple), lactic acid (Source–curd), tartaric acid (Source–tamarind), oxalic acid (Source–tomato), methanoic acid (Source–ant sting, nettle sting), etc.
On the Basis of Ionisation
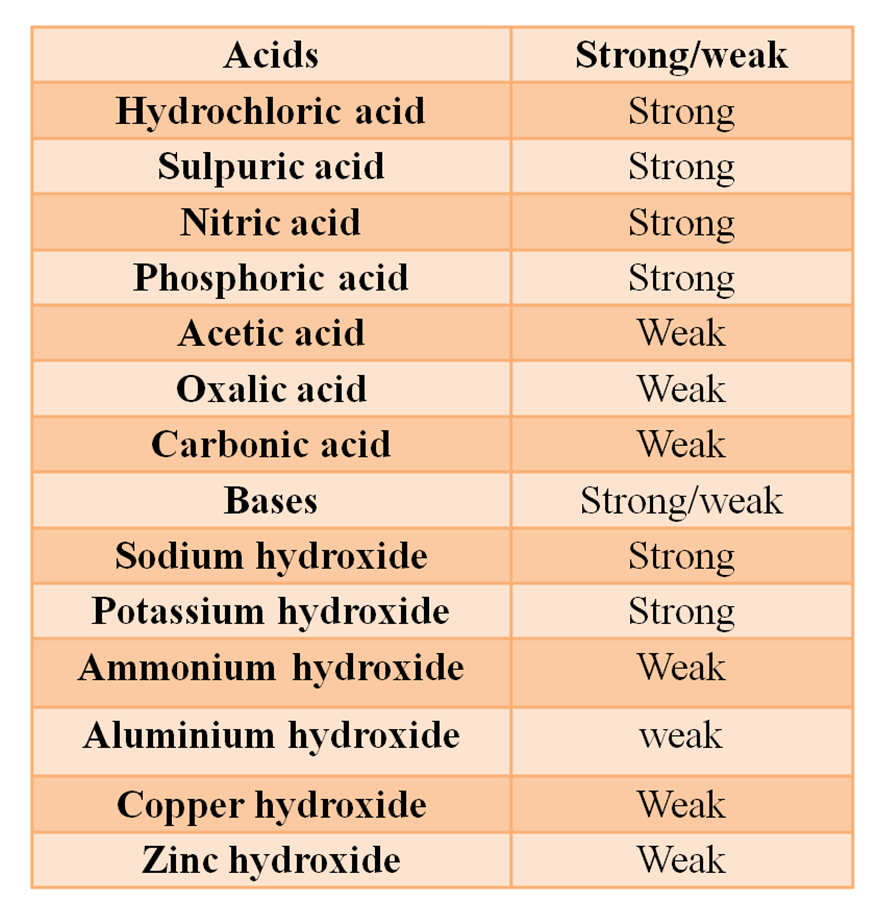
(i):- Strong Acid The acids which ionize almost completely are called strong acids. e.g., mineral acids (except H2CO3).
(ii):- Weak Acid The acids which ionize only partially or to a lesser extent are called weak acids. e.g., organic acids.
On the Basis of the Amount/Concentration of Acid present in Aqueous Solution
(i):- Dilute Acid If in an aqueous solution. Concentration of acid is low, it is called dilute acid.
(ii):- Concentrated Acid If concentration of acid is high, it is called concentrated.
Bases
Substance that furnish hydroxide ions (OH–) in aqueous solution are called bases. Bases are bitter in taste and soapy to touch and turn red litmus solution blue. Their examples are caustic soda or sodium hydroxide NaOH, calcium hydroxide Ca(OH)2, caustic potash or potassium hydroxide KOH, milk of magnesia or magnesium hydroxide Mg(OH)2, etc.
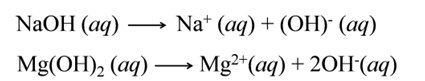
Types of Bases
Bases are of two types:-
(i):- Strong Bases The substances/bases which ionise completely to furnish OH- ions are called strong bases. e.g.,
![]()
(ii):- Weak Bases The bases which ionise only partially are called weak bases. e.g.,
![]()
Alkali
Water soluble base is called alkali. eg., NaOH, KOH, etc. These are usually in laboratory as aqueous solution. These are soapy to touch, bitter and corrosive. Never taste them as they may cause harm. All alkalies are base, but all base, but all base are not alklies.
Strong/Weak Acids and Bases are Tabulated Below
Chemical Properties of Acids
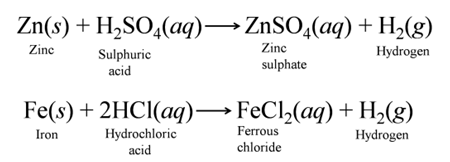
(a) Reaction with Metals Dilute acids (e.g., HCl and H2SO4 not HNO3) react with certain active metals like zinc (Zn), iron (Fe), etc to evolve H2, gas. For Example:-
Test for H2 Gas When a burning match stick is brought near the hydrogen gas, it burns with pop sound.
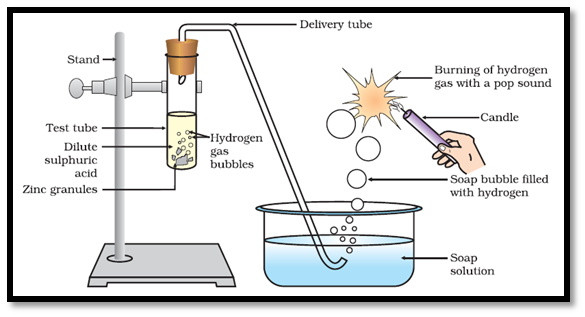
Metal Oxides
When metal reacts with O2 and H2O present in atmosphere it produces metal oxides. Metal oxides are base in nature.
(b) Reaction with Metal Oxides Acids react with certain metal oxides (basic oxides) to form salt and water. For Example:-
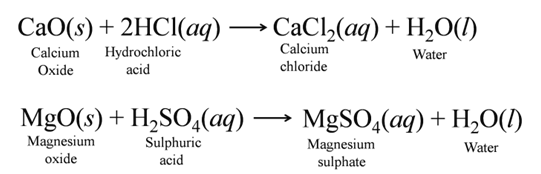
(c) Reaction with Metal Carbonate and Hydrogen Carbonate Acids react with metal carbonates and hydrogen carbonates to produce carbon dioxide gas. 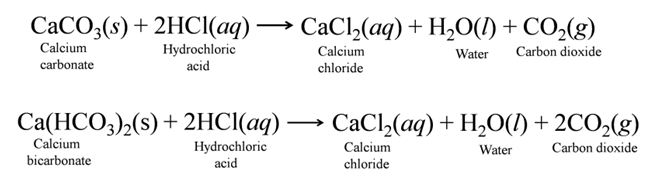
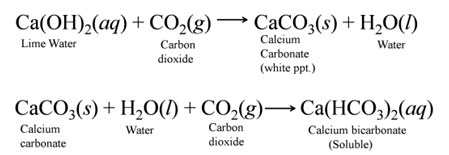
Test for , Gas When gas is passed through lime water, it turns milky due to the formation of white ppt. of CaCO3. But if CO2 is passed in excess, milkiness disappears due to the formation of CaCH(O3)2 which is soluble in water. For Example:-
Chemical Properties of Bases
(a) Reaction with Metals Strong bases react with active metals to produce hydrogen gas.

(b) Reaction with Non-metallic Oxide Bases react with non-metallic oxides (acidic oxides) to produce salt and water. This reaction proves that non-metallic oxides are acidic in nature.
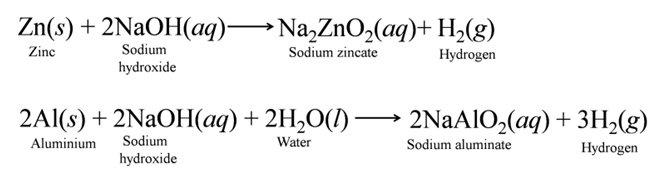
Reaction Between Acids and Bases
Acids react with bases to produce salt and water. In this reaction, acid neutralize a base and the reaction is called neutralization reaction. i.e., reduce its effect or vice-versa, thus the reaction is known as neutralisation reaction.

Common Characteristics of Acids and Bases
Both acids and bases conduct free electric current in their aqueous solutions due to the presence of free ions.
Effect of Dilution on an Acid or Base
Mixing of an acid or base with water is called dilution. It results in decrease in the concentration of ions (H3O+/OH–) per unit volume and the acid or base is said to be diluted.
When water is added to an acid or base, their molecules dissociate to form ions. The H+ ions
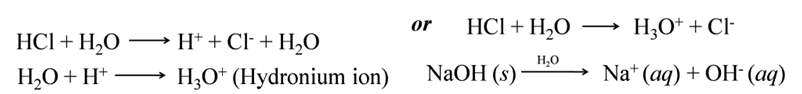
Note:- The process of dissolving an acid or base in water is a highly exothermic reaction. If water is added to a concentrated acid, the heat generated may cause the mixture to splash out and cause burns. To avoid an accident, acid (or base) should be slowly added to the container containing water along with its sides with constant stirring.
Uses of Acids
- Vinegar (acetic acid) is used to preserve pickles and Chinese food.
- In stomach, hydrochloric acid is released which makes the medium acidic and leads to coagulation of proteins. It also helps in digestion and kill the bacteria coming to the stomach along with the food.
- Cold drinks contain carbonic acid.
- Oxalic acid is used to remove ink or rust stains.
- Boric acid is used as an antiseptic and for eye wash.
- Sodium benzoate is used as a preservative in cold drinks.
- Oranges and amla contain ascorbic acid (vitamin C) which prevents scurvy.
Indicators
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Indicators
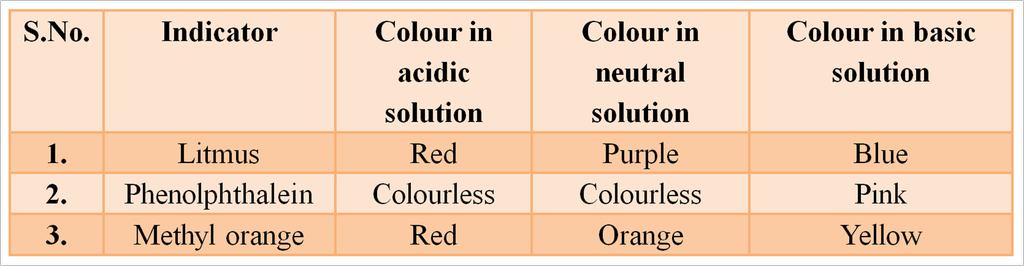
Indicators
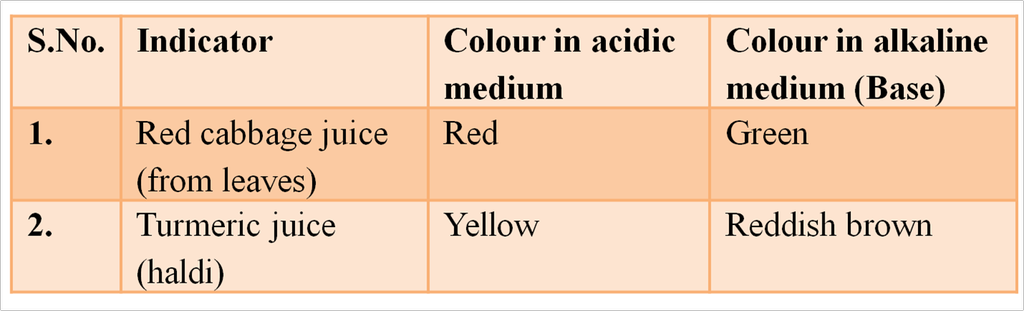
The easiest way to detect whether a solution is acidic, alkaline or neutral is to use an indicator. Indicators are substances that change their colour or odour if they are put into an acid or alkaline solution. Litmus, turmeric and China rose extract, etc are natural indicators.
Some Natural Indicators with Characteristic Colour
Synthetic Indicators
Those chemical substances which change their colour in acids and bases are called synthetic indicators.
They are also called synthetic acid base indicators. e.g., methyl orange, phenolphthalein methylene blue, and methyl red are synthetic indicator.
Some Synthetic Indicators with Characteristic Colour
Olfactory Indicators
Those substances whose odour changes in acidic or basic media are called olfactory indicators.
Vanilla, onion and clove can be used as olfactory indicator.
Litmus Indicators
It is purple dye, which is extracted from lichen, a plant from the division Thallophyta. It is commonly used as acid-base indicator. When the litmus solution is neither acidic nor basic, its colour is purple. When purple solution is acidified, it changes to blue colour whereas it changes to red colour when a small amount of base is added to blue litmus solution.
Strength of an Acid or Base
Strength of an acid or base depends on the number of H+ ions or OH- ions produced by them respectively.
Larger number the of H+ ions produced by an acid, stronger is the acid. Similarly, larger the number the OH- ions produced by a base, stronger is the base.
To judge how strong a given acid or base is, a universal indicator is used which is mixture of several indicators. It shows different colours at different concentrations of hydrogen ion in a solution.
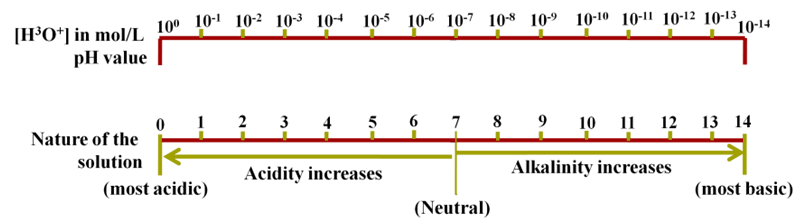
The pH Scale
It is a scale used for measuring hydrogen ion concentration. The p in the pH stands for potenz which means power in German. It has values zero (very acidic) to fourteen (very alkaline).
pH is number which indicates the acidic or basic nature of a solution. Higher the hydronium ion concentration present in the solution lower is their pH value.
{pH means power of hydrogen ions}
- If pH > 7, solution is basic
- If pH < 7, solution is acidic
- If pH = 7, solution is neutral
Importance of pH in Everyday Life
Following are the examples showing importance of pH in everyday life
(i):- pH of Soil Every type of plant requires a specific pH range for healthy growth. Therefore, the nature of soil is known first by testing its pH and then a particular crop is grown in it. It is also suitable for selecting the fertiliser for a particular crop by knowing the pH of he soil.
(ii):- Effect pH on our Digestion HCl present in the stomach helps in the digestion of food. When acidity increases in the stomach, pain, irritation and indigestion is caused. To correct the disturbed pH range, milk of magnesia is used as a medicine, which is also called antacid as it reduces the
effect of acid (or acidity).
(iii):- Effect of pH in Tooth Decay Tooth enamel is made up of calcium phosphate and is the hardest substance in the body. If the pH inside the mouth decreases than 5.5, the decay of tooth enamel begins. This happens when the bacteria present in the mouth work on the left over food particles and produce acid. To prevent tooth decay toothpastes (basic) are used which neutralise the excess acid.
(iv):- When insects like honeybee, ant, etc bite; they leave an acid in the skin, that causes pain and irritation. If a mild base like baking soda is applied on the affected area, it gives relief.
(v):- Stinging Hair of Neetle leaves injects methanoic acid in the skin which causes pain. It is cured by rubbing the affected area with the leaves of dock plant, found in the same locality where the neetle plant is found.
Salts
- Books Name
- Kaysons Academy Chemistry Foundation Book
- Publication
- Kaysons Publication
- Course
- JEE
- Subject
- Chemistry
Salts
Salts are produced by the neutralisation reaction between acid and base.
![]()

Here X is non – metal and M is metal i.e., we can say when metal ion takes place of H - ions, then salt is formed.
Family of Salts
Salts having the same positive or negative radicals are said to belong to a family. For Example:-
NaCl and Na2SO4 belong to the family of sodium salts. Similarly, NaCl and KCl belong to the family of chloride salts.
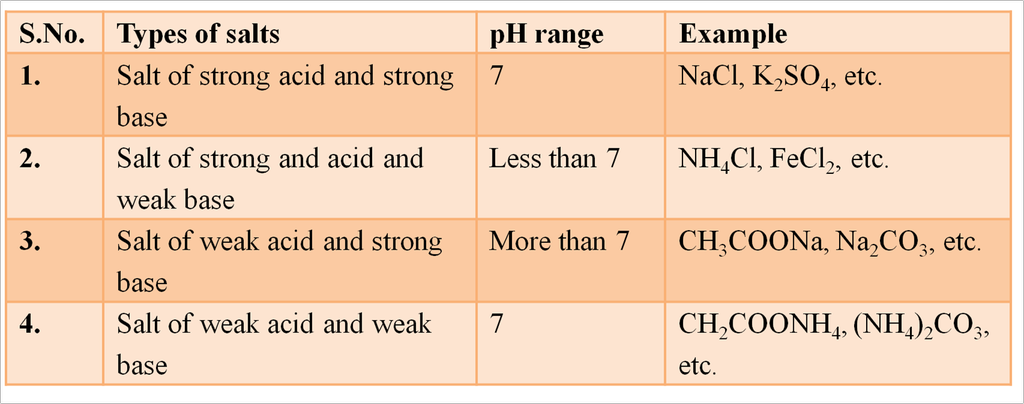
pH of Salts
- Salts of strong acid and a strong base are neutral with pH value of 7.
- Salts of a strong acid and weak base are acidic with pH value less than 7.
- Salts of strong base and weak acid are basic in nature with pH value more than7.
Common Salt: Sodium Chloride
Common salt is formed by the combination of hydrochloric acid and sodium hydroxide solution. It is the salt that we use in food.
It is obtained on large scale from sea water by separating other salts from it. It may also be obtained from rock salt.
Uses of Sodium Chloride
- Common salt, apart from cooking food, is used as a preservative.
- It is also used for manufacture of Na metal and Cl2 (g) by electrolysis in molten state.
- The common salt is an important raw material for various materials of daily use, such as sodium hydroxide, baking soda, washing soda, bleaching power and many more.
Caustic Soda (Sodium Hydroxide [NaOH])
When electricity is passed through an aqueous solution of sodium chloride (called brine), it decomposes to form sodium hydroxide. The process is called the chlor-alkali process because of the products formed chlor for chlorine and alkali for sodium hydroxide.
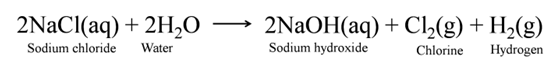
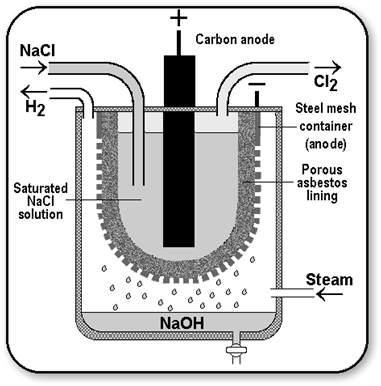
Uses of Hydrogen
- Hydrogen is used in the hydrogen of oils to obtain solid fats. (Vegetable ghee).
- It is used to make ammonia for fertilizers, methanol and production of hydrochloric acid. Hydrochloric acid is used in the preparation of ammonium chlorides, in medicines and cosmetics.
- Liquid hydrogen is used as a fuel for rockets.
Uses of Chlorine
- Chlorine is used to sterlise drinking water supply and the water in swimming pools.
- It is used in the production of bleaching powder, Poly Vinyl Chloride (PVC) pesticides, chloroform, paints and dyestuff.
Uses of Caustic Soda
- It is used for making soaps and detergents.
- It is used in the production of bleaching powder, Poly Vinyl Chloride (PVC) pesticides, chloroform, paints and dyestuff.
Bleaching Powder
(Calcium Oxychloride [CaOCl2])
It is produced by the action of chlorine on dry slaked lime.
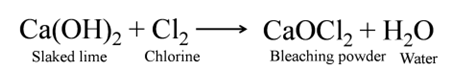
On standing for a longer time, it undergoes auto-oxidation due to which bleaching action decreases. Hypo or sodium bisulphite is used as antichlor during bleaching action.
Uses of Bleaching Powder
- It is used for bleaching purposes in textile and paper industry and in laundry.
- It is also used as a disinfectant for water.
- It is used as an oxidizing agent too.
- It is used in the manufacture of chloroform.
Baking Soda
(Sodium Hydrogen Carbonate or Sodium Bicarbonate [NaHCO3])
The soda commonly used in the kitchen for making tasty crispy pakoras is baking soda. It is the major constituent of baking powder. Sometimes it is added for faster cooking. Chemically it is sodium hydrogen carbonate. It is produced using sodium chloride as one of the raw materials.
Manufacture of baking soda is shown in reaction below
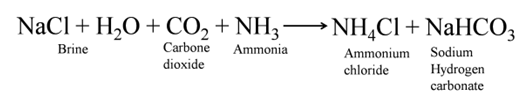
Uses of Baking Soda
For making baking powder, which is a mixture of baking soda (sodium hydrogen carbonate) and a mild edible acid such as tartaric acid. When baking powder is heated or mixed in water, the following reaction takes place
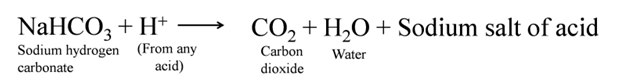
Carbon dioxide produced during the reaction causes bread or cake to rise making them soft and spongy.
Sodium hydrogen carbonate is also an ingredient in antacids. Being alkaline, it neutralises excess acid in the stomach and provides relief.

It is also used in soda-acid fire extinguishers
Washing Soda
(Sodium Carbonate [Na2CO3.10H2O])
Sodium Carbonate can be obtained by heating baking soda; recrystallisation of sodium carbonate gives washing soda. It is also a basic salt.
![]()
Uses of Washing Soda
- It is used in glass, soap and paper industry.
- It is used for the manufacture of sodium compounds like borax.
- It also removes permanent hardness of water.
- It is used as cleansing agent (detergent) in houses and laundries.
Plaster of Paris
(Calcium Sulphate Hemihydrate [CaSO4.1/2H2O])
It is obtained by heating gypsum (CaSO4.2H2O) at 373 K. At this temperature, gypsum loses water molecules and forms plaster of paris.
![]()
When gypsum is heated above 400K, dead burnt plaster (anhydrous CaSO4) is obtained which does not have the property of hardening.
Uses of Plaster Paris
- It is used by doctors for joining the fractured bones in right position i.e., for making plaster to support fractured bones.
- POP made by it is also used for making decorative pieces and for making designs on ceilings.
Water of Crystallization
Crystals of some compounds seem to be dry (or anhydrous), but actually contain some water molecules attached to them. This water is called water of crystallization and such salts are called hydrated salts. Water of crystallization is the fixed number of water molecules present in one formula unit of a salt. For Example:-
- Hydrated copper sulphate (blue vitriol) CaSO4.5H2O
- Hydrated ferrous sulphate (green vitriol) FeSO4.7H2O
- Washing soda crystals Na2CO3.10H2O
This water is removed by heating the crystal of the hydrated salt.

 Kaysons Publication
Kaysons Publication
