1 IMPORTANCE OF CHEMISTRY
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Chemistry
Chapter 1:
SOME BASIC CONCEPTS OF CHEMISTRY
DEVELOPMENT OF CHEMISTRY
Very early chemists were typically intended principally for the accomplishment of a selected goal or product. Creating fragrances and soaps didn't need abundant theory, simply a decent formula and careful attention to detail. There was no customary method of naming materials (and no table that everybody may agree on). However, science developed over the centuries.
Major progress was created in putt chemistry on a solid foundation once Boyle (1637-1691) began his analysis in chemistry. He developed the fundamental concepts for the behaviour of gases; gases may thenceforth be represented mathematically. Boyle conjointly helped pioneer the concept that tiny particles may mix to create molecules. a few years later, John Dalton used these concepts to develop the atomic theory.
The field of science started to grow quickly during the 1700s. Joseph Priestley (1733-1804) separated and described a few gases: oxygen, carbon monoxide, and nitrous oxide. It was subsequently found that nitrous oxide ("chuckling gas") filled in as a sedative. This gas was utilized for that reason without precedent for 1844 during a tooth extraction. Different gases found during that opportunity were chlorine, by C.W. Scheele (1742-1786) and nitrogen, by Antoine Lavoisier (1743-1794). Lavoisier has been viewed as by numerous researchers to be the "father of science".
Scientific experts kept on finding new mixtures during the 1800s. The science likewise started to foster a more hypothetical establishment. John Dalton (1766-1844) set forth his nuclear hypothesis in 1807. This thought permitted researchers to contemplate science in a substantially more orderly manner. Amadeo Avogadro (1776-1856) laid the foundation for a more quantitative way to deal with science by computing the quantity of particles in a given measure of a gas. A great deal of exertion was advanced in concentrating on compound responses. These endeavors prompted new materials being created. Following the innovation of the battery by Alessandro Volta (1745-1827), the field of electrochemistry (both hypothesis and application) created through significant commitments by Humphry Davy (1778-1829) and Michael Faraday (1791-1867). Different region of the discipline additionally advanced quickly.
It would take an enormous book to cover improvements in science during the 20th hundred years and up to the present time. One significant area of development was in the space of the science of living cycles. Research in photosynthesis in plants, the revelation and portrayal of compounds as biochemical impetuses, explanation of the designs of biomolecules like insulin and DNA — these endeavors brought about a blast of data in the field of organic chemistry.
The viable parts of science were not overlooked. Crafted by Volta, Davy, and Faraday at last prompted the improvement of batteries that gave a wellspring of power to control various gadgets.
Charles Goodyear (1800-1860) found the course of vulcanization, permitting rubber to be created for the tires of the relative multitude of vehicles that we have today. Louis Pasteur (1822-1895) spearheaded the utilization of intensity cleansing to dispose of undesirable microorganisms in wine and milk. Alfred Nobel (1833-1896) created explosive. After his passing, the fortune he produced using this item was utilized to finance the Nobel Prizes in science and the humanities. J.W. Hyatt (1837-1920) fostered the primary plastic. Leo Baekeland (1863-1944) fostered the primary engineered gum, which is broadly utilized for economical and solid dinnerware.
IMPORTANCE OF CHEMISTRY
Chemistry is one the most important subject. This subject plays an important role in science and is also related to other branches of science. Being chemistry an important subject it is used in every aspect of a person’s life from the food consumed to the products used. The improvement of chemical science has altered the premise of current medication. With ever-increasing research in chemistry, wonder drugs like penicillin and streptomycin have been developed. Not only in medicine but chemistry is also applied in many different areas of science and technology. It is applied in agriculture, supply of food, contribution to better hygiene and sanitation, saving the environment, increase in comfort, pleasure and luxuries, transport and communication, and atomic energy.
2. NATURE OF MATTER
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Chemistry
STATES OF MATTER
On the basis of physical properties, matter may be classified into the following three states :
- Solid State
- Liquid State
- Gaseous State
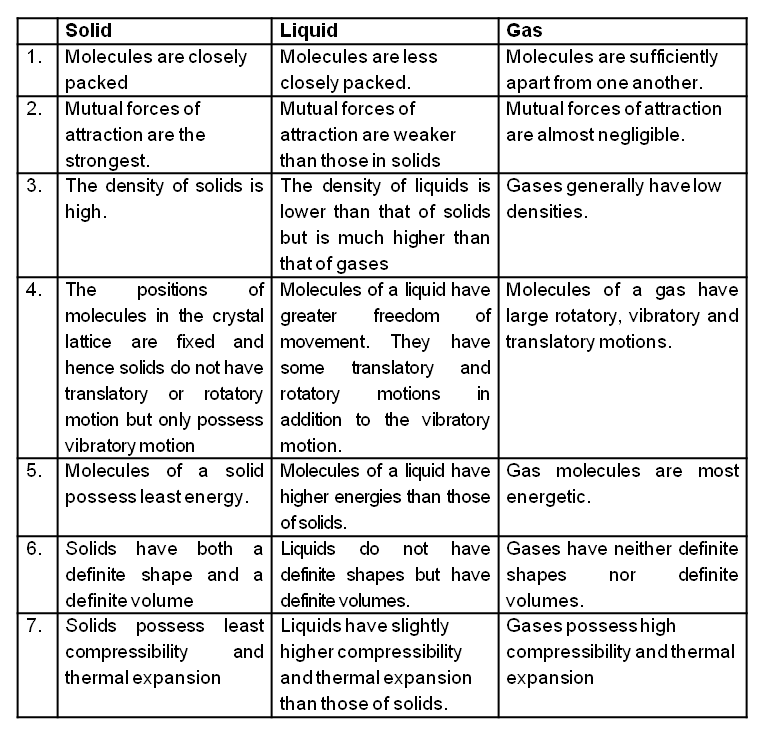
Comparison of the characteristics of a solid, a liquid and a gas
Gases
The molecules of a gas are free to move in all directions. The space in between them is very large. The gaseous molecules possess motion of all three types, namely translational, rotational and vibrational. The molecular forces of attraction between gases are very much weak. Hence a gas has neither a definite shape nor a definite volume.
- Gases are highly compressible
- Gases expand without limits
- Gases exert pressure on the walls of the container uniformly in all directions.
- Gases diffuse rapidly through each other to form a homogeneous mixture.
6. DALTON’S ATOMIC THEORY
- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Chemistry
DALTON LAW OF PARTIAL PRESSURE
At a given temperature, the total pressure exerted by two or more non-reacting gases occupying a definite volume is equal to the sum of the partial pressures of the component gases. Mathematically,
P = pA + pB + pC + …..
when P is the total pressure and pA, pB, pC, ….. are the partial pressures of the component gases A, B, C, ….. respectively. The pressure that a component gas of the gaseous mixture would exert if it were only present in the volume under consideration at a given temperature, is the partial pressure of the component.
Derivation of Dalton’s Law
Let n1 and n2 be the no. of moles of two non-reacting gases ‘A’ and ‘B’ filled in a vessel of volume ‘V’ at temperature T.
Total pressure in the vessel ‘P’ may be calculated as
PV = (n1 + n2)RT …….(i)
Individual or partial pressure may be calculated as,
pAV = n1RT …….(ii)
pBV = n2RT …….(iii)
Adding (ii) and (iii), we get
(pA + pB)V = (n1 + n2)RT …….(iv)
Comparing equations (i) and (iv), we get
P = pA + pB (Dalton’ expression)
Dividing equation (ii) by (i), we get
![]() = xA
= xA
pA = xA ´ P
where xA = mole fraction of ‘A’. Similarly, dividing (iii) by (i), we get
pB = xB ´ P
i.e., Partial pressure of a component = Mole fraction ´ total pressure
Relationship between total pressure and individual pressures (before mixing) of the constituent gases at constant temperature
At constant temperature, let V1 volume of a gas A at a pressure p1 be mixed with V2 volume of gas B at a pressure p2. Both these gases do not react chemically.
Total volume = V1 + V2
Let the total pressure be P and partial pressures of A and B be pA and pB respectively. Applying Boyle’s law,
PA(V1 + V2) = p1V1 …….(i)
and pB(V1 + V2) = p2V2 …….(ii)
Adding (i) and (ii)
pA + pB = 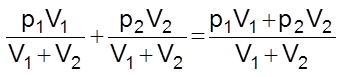
or P = 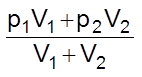
Dalton’s Law fails in those gases which react chemically so not applicable to the following mixtures.
(i) NH3+HCl
(ii) NO +O2
(iii) H2+F2
(iv) H2+Cl2
(v) SO2+ Cl2
Applications
(i) In the determination of pressure of dry gas : When a gas is called over water, then it is moist , water also vaporizes simultaneously and exerts its own partial pressure. Partial pressure of water is called its aqueous tension. It depends upon temperature.
If P and P¢ are the pressure of the dry gas and the moist gas respectively at t°C and p is the aqueous tension at that temperature, then by Dalton’s Law of Partial Pressures
P = P¢ - p
(ii) In the calculation of partial pressures : In a mixture of non-reacting gases A, B, C etc., if each gas is considered to be an ideal gas, then applying PV = nRT
PA = nA ![]() , pB = nB
, pB = nB ![]() pC = nC
pC = nC ![]()
And so on.
By Dalton’s law of partial pressures,
Total pressure, P = pA + pB + pC + … = ![]() (nA + nB + nC + …)
(nA + nB + nC + …)
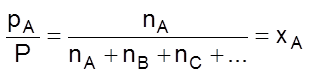 (mole fraction of A)
(mole fraction of A)
or pA = xA ´ P
Similarly, pB = xB ´ P and so on. Thus
Partial pressure of A = Mole fraction of A ´ Total pressure
Amagat Law of Partial volume
Total volume of a mixture of gases which do not react at constant temperature and pressure is equal to sum of individual volumes (partial volumes) of constituent gases.
V = åVi = V1 + V2 + V3 + ….. + Vn

 Ritan Sheth
Ritan Sheth
