1. THERMODYNAMIC TERMS
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
CHAPER – 6 THERMODINAMICS
IMPORTANT TERMS AND DEFINITIONS
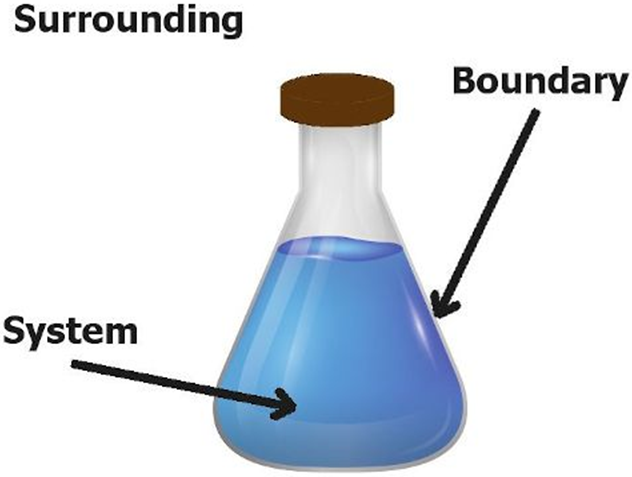
System: Refers to the portion of universe which is under observation.
Surroundings: Everything else in the universe except system is called surroundings. The Universe = The System + The Surroundings.
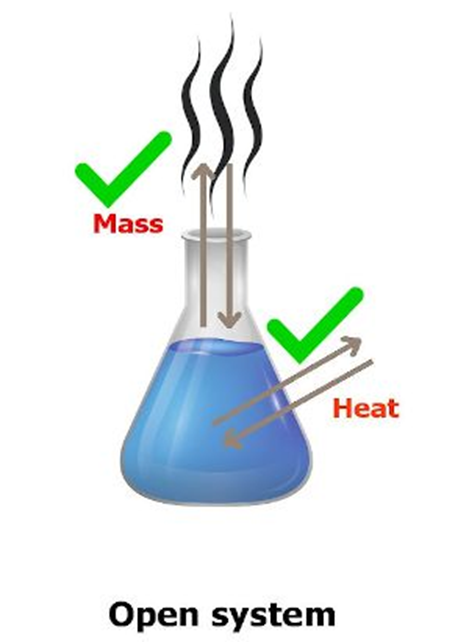
Open System: In a system, when there is exchange of energy and matter taking place with
the surroundings, then it is called an open system.
For Example: Presence of reactants in an open beaker is an example of an open system. Closed System: A system is said to be a closed system when there is no exchange of matter ‘ but exchange of energy is possible.
For example: The presence of reactants in a closed vessel made of conducting material.
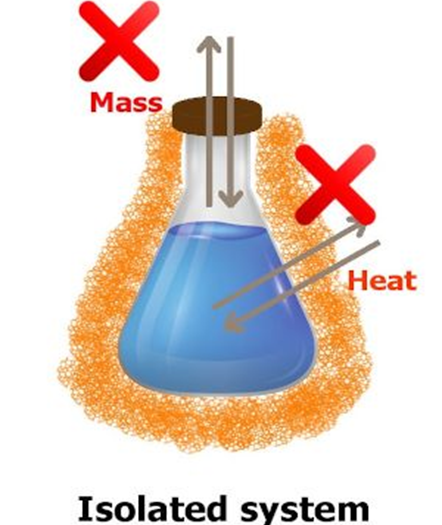
Isolated System: In a system, when no exchange of energy or matter takes place with the surroundings, is called isolated system.
For example: The presence of reactants in a thermoflask, or substance in an insulated closed vessel is an example of isolated system.
Homogeneous System: A system is said to be homogeneous when all the constituents present is in the same phase and is uniform throughout the system.
For example: A- mixture of two miscible liquids.
Heterogeneous system: A mixture is said to be heterogeneous when it consists of two or more phases and the composition is not uniform.
For example: A mixture of insoluble solid in water. ’
The state of the system: The state of a thermodynamic system means its macroscopic or bulk properties which can be described by state variables:
Pressure (P), volume (V), temperature (T) and amount (n) etc.
They are also known as state functions.
Isothermal process: When the operation is carried out at constant temperature, the process is said to be isothermal. For isothermal process, dT = 0 Where dT is the change in temperature.
Adiabatic process: It is a process in which no transfer of heat between system and surroundings, takes place.
Isobaric process: When the process is carried out at constant pressure, it is said to be isobaric. i.e. dP = 0
Isochoric process: A process when carried out at constant volume, it is known as isochoric in nature.
Cyclic process: If a system undergoes a series of changes and finally returns to its initial state, it is said to be cyclic process.
Reversible Process: When in a process, a change is brought in such a way that the process could, at any moment, be reversed by an infinitesimal change. The change r is called reversible.
•Internal Energy
It is the sum of all the forms of energies that a system can possess.
In thermodynamics, it is denoted by AM which may change, when
— Heat passes into or out of the system
— Work is done on or by the system
— Matter enters or leaves the system.
Change in Internal Energy by Doing Work
Let us bring the change in the internal energy by doing work.
Let the initial state of the system is state A and Temp. TA Internal energy = uA
On doing’some mechanical work the new state is called state B and the temp. TB. It is found to be
TB > TA
uB is the internal energy after change.
∴ Δu = uB – uA
Change in Internal Energy by Transfer of Heat
Internal energy of a system can be changed by the transfer of heat from the surroundings to the system without doing work.
Δu = q
Where q is the heat absorbed by the system. It can be measured in terms of temperature difference.
q is +ve when heat is transferred from the surroundings to the system. q is -ve when heat is transferred from system to surroundings.
When change of state is done both by doing work and transfer of heat.
Δu = q + w
First law of thermodynamics (Law of Conservation of Energy). It states that, energy can neither be created nor be destroyed. The energy of an isolated system is constant.
Δu = q + w.
2. APPLICATIONS
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
APPLICATIONS
• Work (Pressure-volume Work)
Let us consider a cylinder which contains one mole of an ideal gas in which a frictionless piston is fitted.
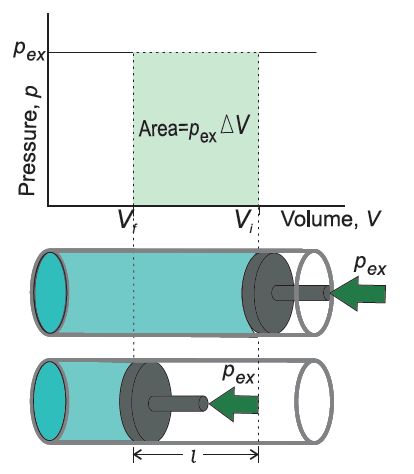
• Work Done in Isothermal and Reversible Expansion of Ideal Gas
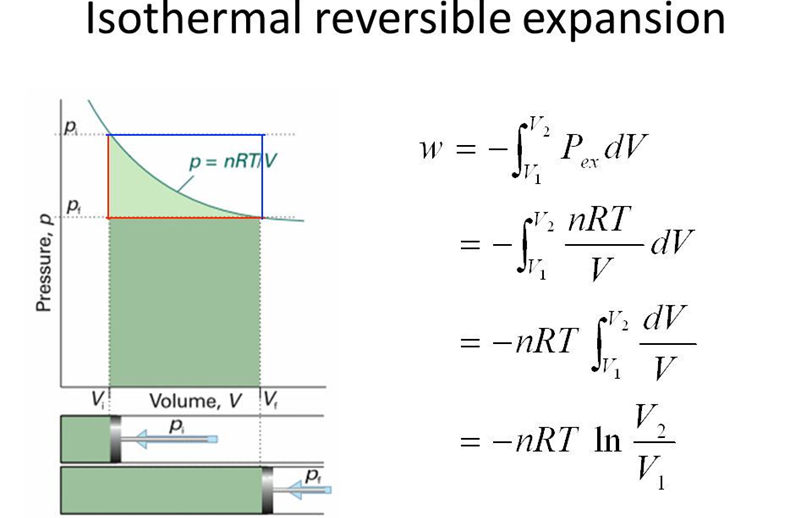
• Isothermal and Free Expansion of an Ideal Gas
For isothermal (T = constant) expansion of an
ideal gas into vacuum ; w = 0 since pex = 0.
Also, Joule determined experimentally that
q = 0; therefore, ∆U = 0
• Enthalpy (H)
It is defined as total heat content of the system. It is equal to the sum of internal energy and pressure-volume work.
Mathematically, H = U + PV
Change in enthalpy: Change in enthalpy is the heat absorbed or evolved by the system at constant pressure.
ΔH = qp
For exothermic reaction (System loses energy to Surroundings),
ΔH and qp both are -Ve.
For endothermic reaction (System absorbs energy from the Surroundings).
ΔH and qp both are +Ve.
Relation between ΔH and Δu.
![]() B
B
Let HA be the enthalpy of reactant A and HB be that of the products.
∴ HA = UA + PVA
HB = UB + PVB
ΔH = HB - HA
= (UB + PVB) – (UA + PVA)
ΔH = ΔU + PΔV (HB – HA)
ΔH = ΔU + PΔV
At constant pressure and temperature using ideal gas law,
PVA = nA RT (for reactant A)
PVB = nB RT (for reactant B)
Thus, PVB – PVA = nB RT - nA RT
= ( nB – nA) RT
PΔV = Δng RT
∴ ΔH = ΔU + Δng RT
• Extensive property
An extensive property is a property whose value depends on the quantity or size of matter present in the system.
For example: Mass, volume, enthalpy etc. are known as extensive property.
• Intensive property
Intensive properties do not depend upon the size of the matter or quantity of the matter present in the system.
For example: temperature, density, pressure etc. are called intensive properties.
• Heat capacity
The increase in temperature is proportional to the heat transferred.
q = coeff. x ΔT
q = CΔT
Where, coefficient C is called the heat capacity.
C is directly proportional to the amount of substance.
Cm = C/n
It is the heat capacity for 1 mole of the substance.
• Molar heat capacity
It is defined as the quantity of heat required to raise the temperature of a substance by 1° (kelvin or Celsius).
• Specific Heat Capacity
It is defined as the heat required to raise the temperature of one unit mass of a substance by 1° (kelvin or Celsius).
q = C x m x ΔT
where m = mass of the substance
ΔT = rise in temperature.
• Relation Between Cp and Cv for an Ideal Gas
At constant volume heat capacity = Cv
At constant pressure heat capacity = Cp
At constant volume qv= CvΔT = ΔU
At constant pressure qp = Cp ΔT = ΔH
For one mole of an ideal gas
ΔH = ΔU + Δ (PV) = ΔU + Δ (RT)
ΔH = ΔU + RΔT
On substituting the values of ΔH and Δu, the equation is modified as
Cp ΔT = CvΔT + RΔT
or Cp-Cv = R
3. MEASUREMENT OF ∆U AND ∆H: CALORIMETRY
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
MEASUREMENT OF ΔU AND ΔH—CALORIMETRY
Determination of ΔU: ΔU is measured in a special type of calorimeter, called bomb calorimeter.
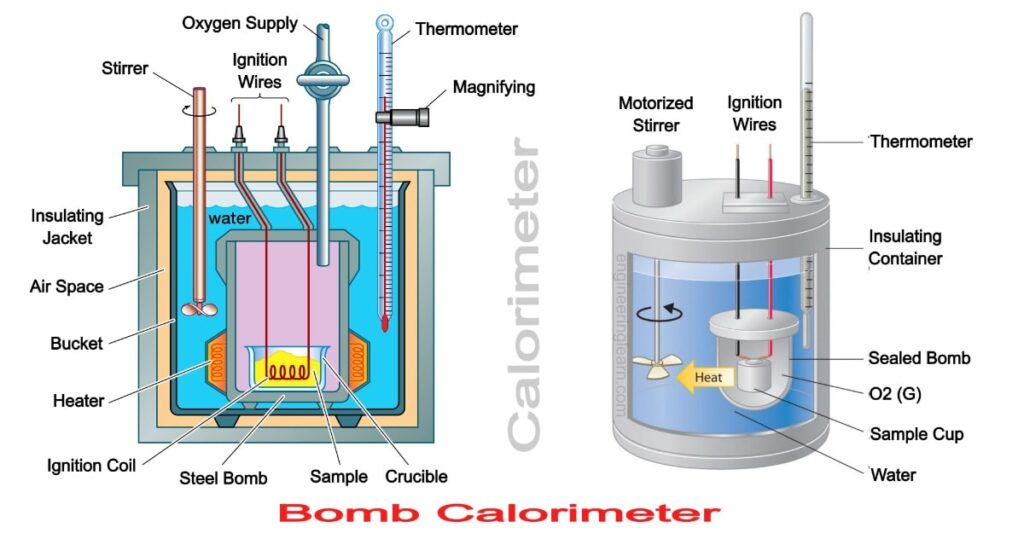
Working with calorimeter. The calorimeter consists of a strong vessel called (bomb) which can withstand very high pressure. It is surrounded by a water bath to ensure that no heat is lost to the surroundings.
Procedure: A known mass of the combustible substance is burnt in the pressure of pure dioxygen in the steel bomb. Heat evolved during the reaction is transferred to the water and its temperature is monitored.
4. ENTHALPY CHANGE, ∆rH OF A REACTION – REACTION ENTHALPY
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ENTHALPY CHANGE, ∆rH OF A REACTION – REACTION ENTHALPY
• Enthalpy Changes During Phase Transformation
Enthalpy of fusion: Enthalpy of fusion is the heat energy or change in enthalpy when one mole of a solid at its melting point is converted into liquid state.
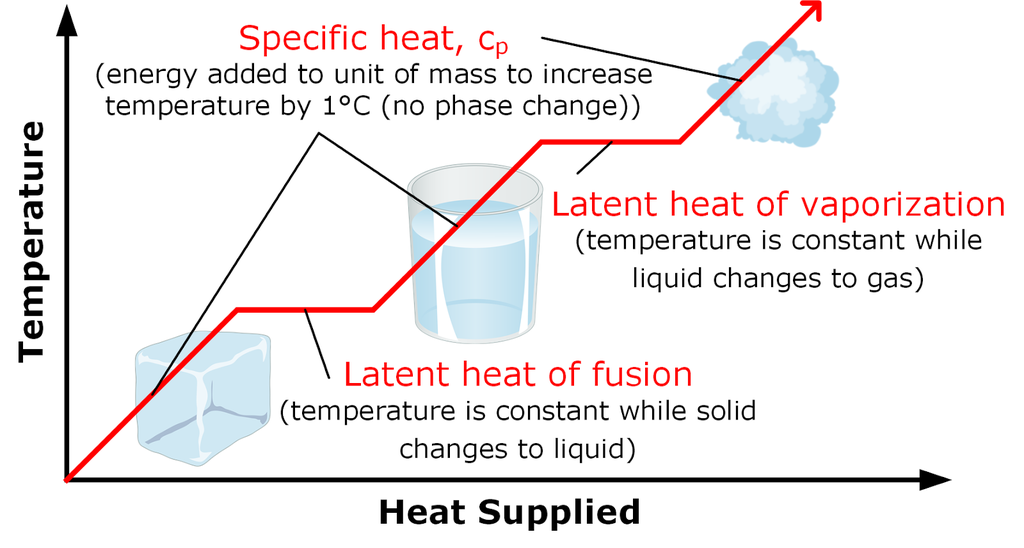
Enthalpy of vaporisation: It is defined as the heat energy or change in enthalpy when one mole of a liquid at its boiling point changes to gaseous state.
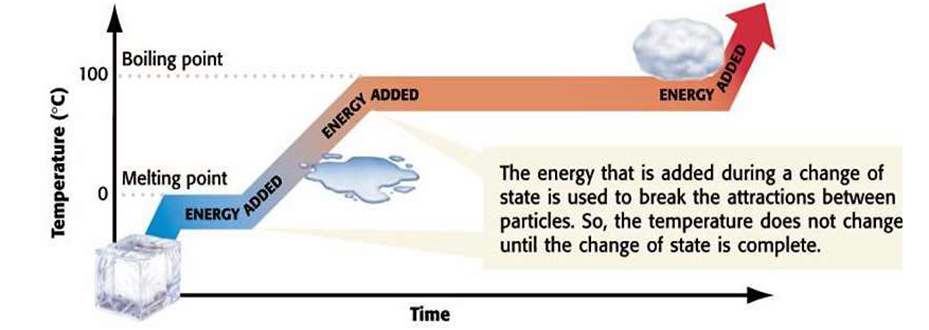
Enthalpy of Sublimation: Enthalpy of sublimation is defined as the change in heat energy or change in enthalpy when one mole of solid directly changes into gaseous state at a temperature below its melting point.
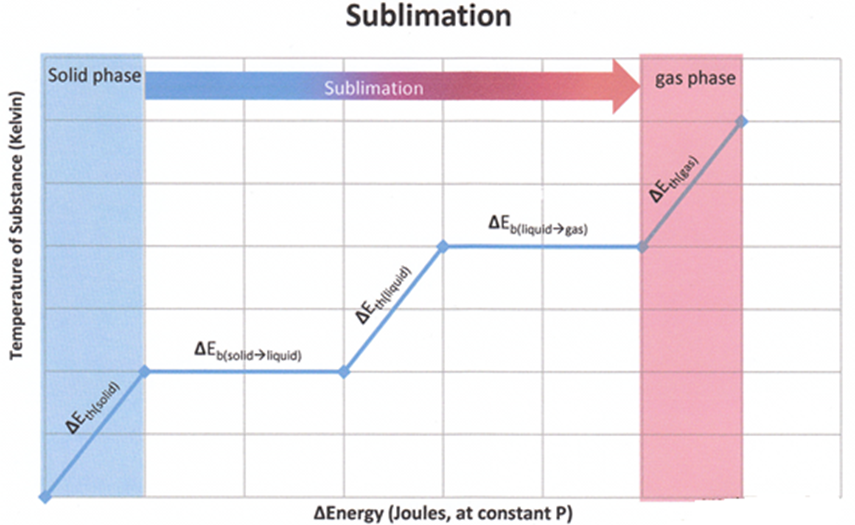
5. ENTHALPIES FOR DIFFERENT TYPES OF REACTIONS
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ENTHALPIES FOR DIFFERENT TYPES OF REACTIONS
• Standard Enthalpy of Formation
Enthalpy of formation is defined as the change in enthalpy in the formation of 1 mole of a substance from its constituting elements under standard conditions of temperature at 298K and 1 atm pressure.
ΔHrº (298.15 K) and ΔGrº (298.15 K)
ΔGrº (T)
ΔHrº (T) and Kp (298.15 K)
Kp (T1) and Kp (T2) where T1, T2 ≠ T
ΔHrº (T) and ΔGrº (298.15 K)
Enthalpy of Combustion: It is defined as the heat energy or change in enthalpy that accompanies the combustion of 1 mole of a substance in excess of air or oxygen.
CO(g) + ½ O2(g) ![]() CO2(g) ΔHº = -283 kJ
CO2(g) ΔHº = -283 kJ
• Thermochemical Equation
A balanced chemical equation together with the value of ΔrH and the physical state of reactants and products is known as thermochemical equation.
Thermochemical equations
- A typical chemical equation is
![]() SO2
SO2
- It is called a thermochemical equation when we add information about ΔH…
![]() SO2 ΔH = -296.9 kJ
SO2 ΔH = -296.9 kJ
- If we change the equation, then the ΔH also changes…
![]() S + O2 ΔH = +296.9 kJ
S + O2 ΔH = +296.9 kJ
- If the reaction is reversed the sign is reversed
- Also, if numbers in the equation change, so will the amount of energy produced/absorbed:
![]() 2SO2 ΔH = -593.8 kJ
2SO2 ΔH = -593.8 kJ
Conventions regarding thermochemical equations
1. The coefficients in a balanced thermochemical equation refer to the number of moles of reactants and products involved in the reaction.
If the coefficients of the chemical equation are multiplied by some factor, the enthalpy change must also be multiplied by the same factor (ΔH is an extensive property).
H2(g) + ½ O2(g) ![]() H2O(l) ΔH = -286 kJ
H2O(l) ΔH = -286 kJ
Multiply by a factor of two:
2H2(g) + O2(g) ![]() 2H2O(l) ΔH = 2 x (-286kJ) = -572kJ
2H2O(l) ΔH = 2 x (-286kJ) = -572kJ
Multiply by a factor of one-half:
½ H2(g) + ¼ O2(g) ![]() ½ H2O(l) ΔH = ½ x (-286kJ) = -134kJ
½ H2O(l) ΔH = ½ x (-286kJ) = -134kJ
• Hess’s Law of Constant Heat Summation
The total amount of heat evolved or absorbed in a reaction is same whether the reaction takes place in one step or in number of steps.
The law stats that the change in enthalpy for a reaction is the same whether the reaction takes place in one or a series of step. The Hess’s law can also be states as the enthalpy change for a chemical reaction is the same regardless of the path by which the reaction occurs.
For example, consider following two paths for the preparation of methylene chloride
Path I:
CH4 (g) + 2 Cl2(g) ![]() CH2Cl2(g) + 2HCl(g) ΔH1º = - 202.3kJ
CH2Cl2(g) + 2HCl(g) ΔH1º = - 202.3kJ
Path II:
CH4 (g) + Cl2(g) ![]() CH3Cl2(g) + HCl(g) ΔH2º = - 98.3kJ
CH3Cl2(g) + HCl(g) ΔH2º = - 98.3kJ
CH3Cl(g) + Cl2(g) ![]() CH2Cl2(g) + HCl(g) ΔH3º = - 104.0kJ
CH2Cl2(g) + HCl(g) ΔH3º = - 104.0kJ
Adding two steps
CH4(g) + 2Cl2(g) ![]() CH2Cl2(g) + 2HCl(g) ΔHº = - 202.3kJ
CH2Cl2(g) + 2HCl(g) ΔHº = - 202.3kJ
Thus whether we follow path 1 or path 2 the enthalpy change of the reaction is same.
ΔH1º = ΔH2º + ΔH3º = - 202.3kJ
• Born-Haber Cycle
It is not possible to determine the Lattice enthalpy of ionic compound by direct experiment. Thus, it can be calculated by following steps. The diagrams which show these steps is known as Born-Haber Cycle.
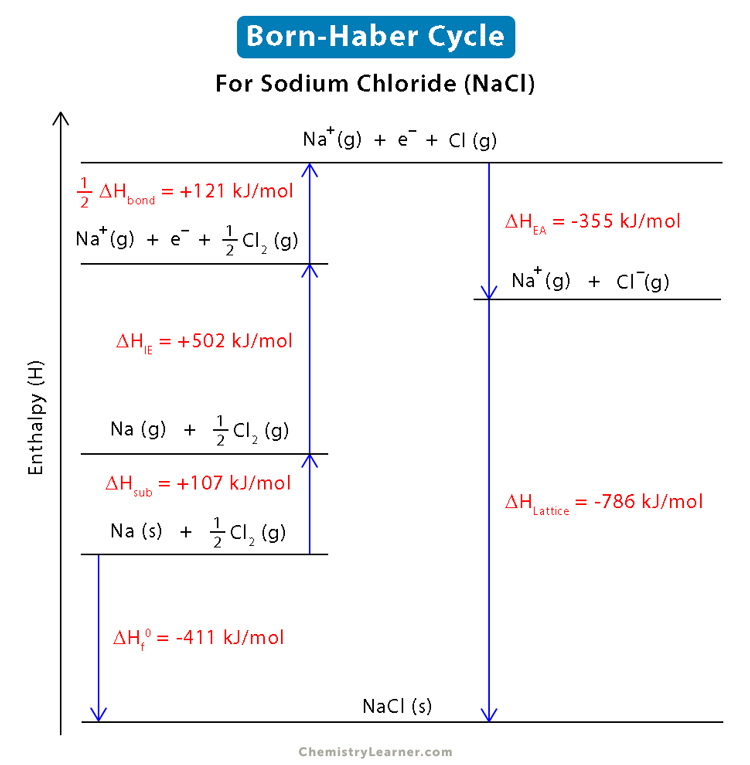
6. SPONTANEITY
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
SPONTANEITY
Spontaneous Process: A process which can take place by itself or has a tendency to take place is called spontaneous process.
Spontaneous process need not be instantaneous. Its actual speed can vary from very slow to quite fast.
A few examples of spontaneous process are:
(i) Common salt dissolves in water of its own.
(ii) Carbon monoxide is oxidised to carbon dioxide of its own.
• Entropy (S)
The entropy is a measure of degree of randomness or disorder of a system. Entropy of a substance is minimum in solid state while it is maximum in gaseous state.
The change in entropy in a spontaneous process is expressed as ΔS
ΔS = ΔSsystem + ΔSsurroundings
ΔS = Overall change in entropy
ΔSsystem = Change in entropy of the system
ΔSsurroundings =Change in entropy of the surroundings
ΔS > 0, + entropy change, reaction is spontaneous
ΔS < 0, - entropy change, reaction is nonspontaneous
Equation for the total change in entropy
• Gibbs Energy and Spontaneity
A new thermodynamic function, the Gibbs energy or Gibbs function G, can be defined as G = H-TS
ΔG = ΔH – TΔS
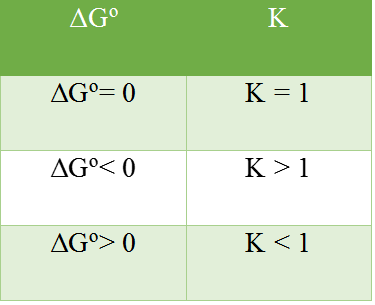
Gibbs energy change = enthalpy change – temperature x entropy change ΔG gives a criteria for spontaneity at constant pressure and temperature, (i) If ΔG is negative (< 0) the process is spontaneous.
(ii) If ΔG is positive (> 0) the process is non-spontaneous.
• Free Energy Change in Reversible Reaction
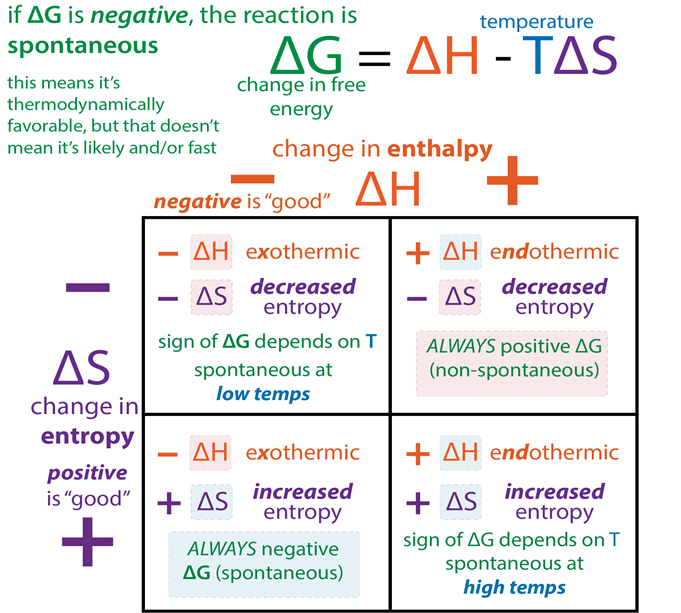
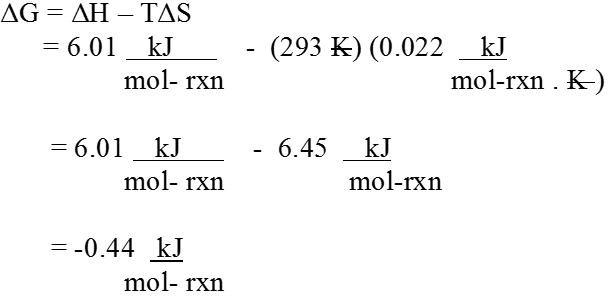
qualitative relationship between the change in standard free energy and the equilibrium constant for a given relation
7. GIBBS ENERGY CHANGE AND EQUILIBRIUM
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
GIBBS ENERGY CHANGE AND EQUILIBRIUM
At equilibrium, i.e., ΔG = 0, the process is neither spontaneous nor non-spontaneous because it is balanced between spontaneous and non-spontaneous behavior. (+ΔH)
So,
ΔG = ΔH – T ΔS = 0
Hence,
ΔH = TΔS or T = ΔH / ΔS
T is the temperature at which the transition from spontaneous to non-spontaneous behaviour happens. T is calculated on the assumption that ΔH and ΔS are temperature independent. In reality, ΔH and ΔS change with temperature. However, for modest temperature changes, the variance in them will not add considerable mistakes.
ΔG and Equilibrium constant
All of the substances (reactants and products) in a chemical reaction may not be in their normal forms. As a result of the connection, the change in Gibbs energy of a reaction is related to the change in standard Gibbs energy.
ΔG = ΔG° + RT ln Q
where:
ΔG° is the standard Gibbs energy change (change in Gibbs energy when all the substances are in their standard state).
Q is the reaction quotient.
The expression of the reaction quotient is similar to that of the equilibrium constant, but there is one single difference between them, i.e., Equilibrium concentrations or partial pressures of products and reactants are included in the equilibrium constant. Whereas Q is expressed in terms of reactant beginning concentration partial pressures and product final concentrations or pressures.
For Example, consider the below example:
aA +bB ⇢ cC + dD
For the above reaction, the reaction Quotient is given by
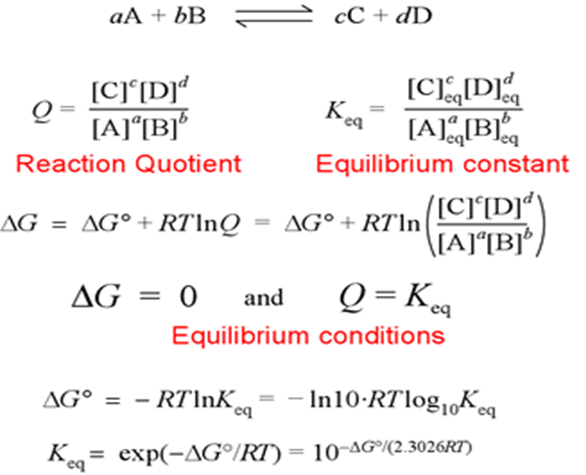
When the values of concentration or partial pressure are other than equilibrium values. When the reaction reaches equilibrium, the concentrations and partial pressure reach their equilibrium values and at this stage, Q = K. At equilibrium, ΔG = 0 and Q = K, then the standard Gibbs energy equation becomes,
0 = ΔG° + RT ln K
Hence,
ΔG° = -RT ln K = -2.303RT log10K
This equation gives the relationship between standard Gibbs energy change for the reaction and its equilibrium constant.

 Ritan Sheth
Ritan Sheth
