1. Classification of elements
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
CHAPTER - 3
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
WHY DO WE NEED TO CLASSIFY ELEMENTS ?
The elements are the basic units of all types of matter. In 1800, only 31 elements were known. By 1865, the number of identified elements had more than doubled to 63. At present 114 elements are known. Of them, the recently discovered elements are man-made. Efforts to synthesise new elements are continuing. With such a large number of elements it is very difficult to study individually the chemistry of all these elements and their innumerable compounds individually. To ease out this problem, scientists searched for a systematic way to organise their knowledge by classifying the elements. Not only that it would rationalize known chemical facts about elements, but even predict new ones for undertaking further study.
2. Genesis of periodic classification
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
GENESIS OF PERIODIC CLASSIFICATION DOBEREINER’S TRIADS
In 1829, Dobereiner arranged certain elements with similar properties in groups of three in such a way that the atomic mass of the middle element was nearly the same as the average atomic masses of the first and the third elements. A few triads proposed by him are listed.
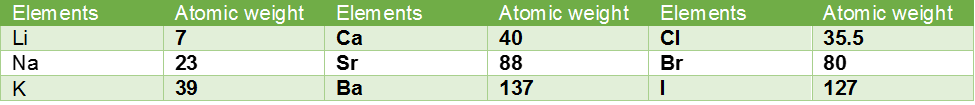
Limitations of Dobereiner’s Trids
The triads given by Dobereiner were helpful in grouping some elements with similar characteristics together, but he could not arrange all the elements known at that time into triads.
• Newlands’ Law of Octaves
John Newlands proposed the law of octaves by stating that when elements are arranged in order of increasing atomic masses, every eighth element has properties similar to the first. Newlands called it law of octaves because similar relationship exists in the musical notes also.
This can be illustrated as
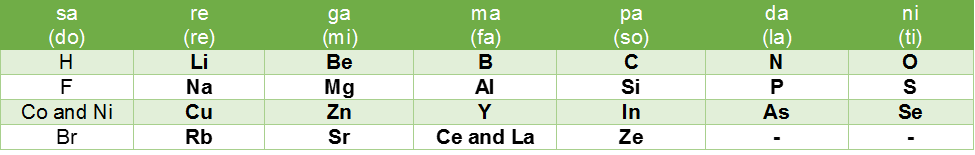
Limitations of Newlands’ Law of Octaves
(i) This classification was successful only up to the element calcium. After that, every eighth element did not possess the same properties as the element lying above it in the same group.
(ii) When noble gas elements were discovered at a later stage, their inclusion in the table disturbed the entire arrangement.
• Mendeleev’s Periodic Table
Mendeleev’s Periodic Law: The physical and chemical properties of the elements are a periodic
function of their atomic masses.
Mendeleev arranged the elements known at that time in order of increasing atomic masses
and this arrangement was called periodic table.
Elements with similar characteristics were present in vertical rows called groups. The horizontal
rows were known as periods.
Description of Mendeleev’s Periodic Table
(i) In the periodic table, the elements are arranged in vertical rows called groups and horizontal rows known as periods.
(ii) There are nine groups indicated by Roman Numerals as I, II, III, IV, V, VI, VII, VIII and zero. Group VIII consists of nine elements which are arranged in three triads. The zero group contains elements belonging to inert gases or noble gases and elements present have zero valency.
(iii) There are seven periods (numbered from 1 to 7) or, horizontal rows in the Mendeleev’s periodic table.
Importance of Mendeleev’s Periodic Table
(i) This made the study of the elements quite systematic in the sense that if the properties of one element in a particular group are known, those of others can be pridicted.
(ii) This helped to a great extent in the discovery of these elements at a later stage.
(iii) Mendeleev corrected the atomic masses of certain elements with the help of their expected positions and properties.
Defects in Mendeleev’s Periodic Table
(i) Hydrogen has been placed in group IA along with alkali metals. But it also resembles halogens of group VII A in many properties. Thus, its position is the Mendeleev’s periodic table is controversial.
(ii) Although the elements in the Mendeleev’s periodic table have been arranged in order of their atomic masses, but in some cases the element with higher atomic mass precedes the element with lower atomic mass.
(iii) We know that the isotopes of an element have different atomic masses but same atomic number. Since, periodic table has been framed on the basis of increasing atomic masses of the elements, different positions must have been allotted to all the isotopes of a particular element.
(iv) According to Mendeleev, the elements placed in the same group must resemble in their properties. But there is no similarity among the elements in the two sub-groups of a particular group.
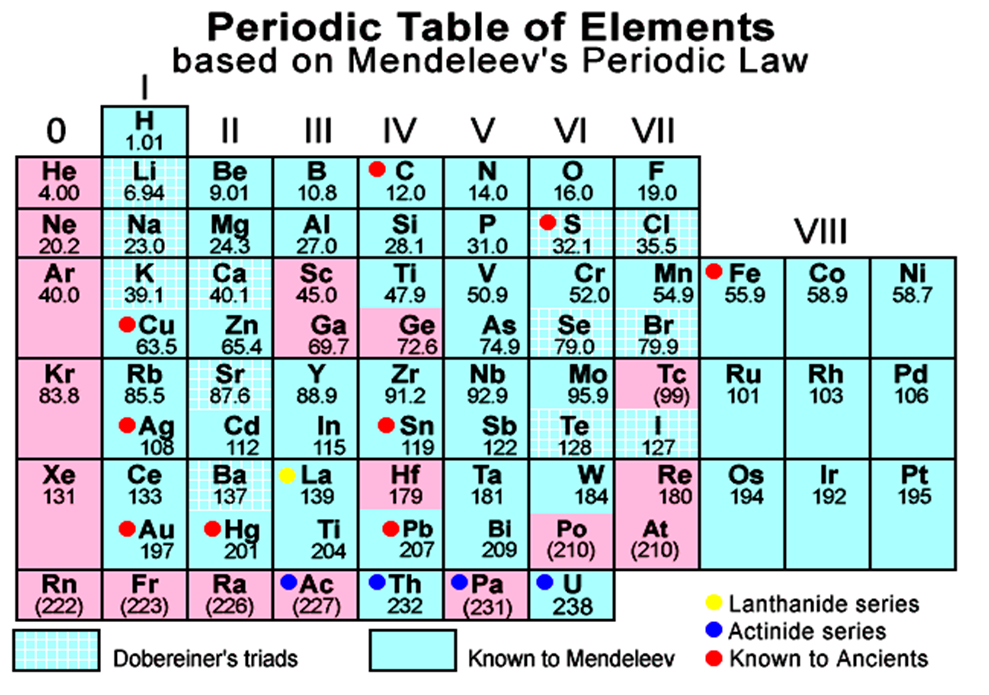
(v) In some cases, elements with similar properties have been placed in different groups.
(vi) Lanthanoids and actinoids were placed in two separate rows at the bottom of the periodic table without assigning a proper reason.
(vii) No proper explanation has been offered for the fact that why the elements placed in group show resemblance in their properties.
3. Modern periodic law
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
MODERN PERIODIC LAW
Physical and chemical properties of the elements are the periodic function of their atomic numbers.
• Present Form of the Periodic Table (Long form of Periodic Table)
The long form of periodic table, also called Modem Periodic Table, is based on Modern periodic law. In this table, the elements have been arranged in order of increasing atomic numbers.
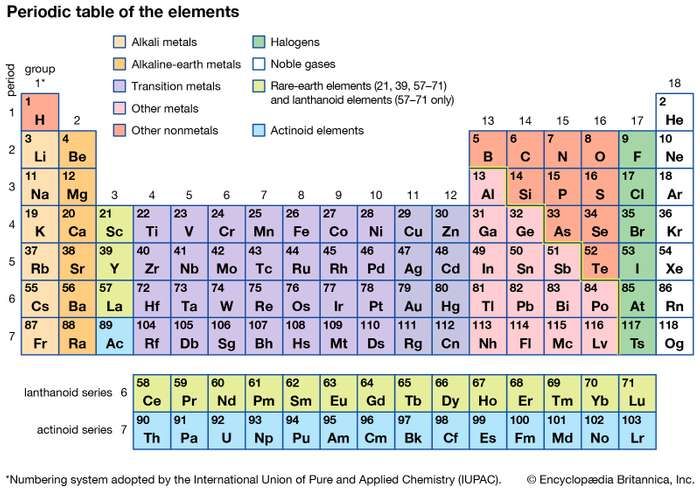
4. Nomenclature of elements
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
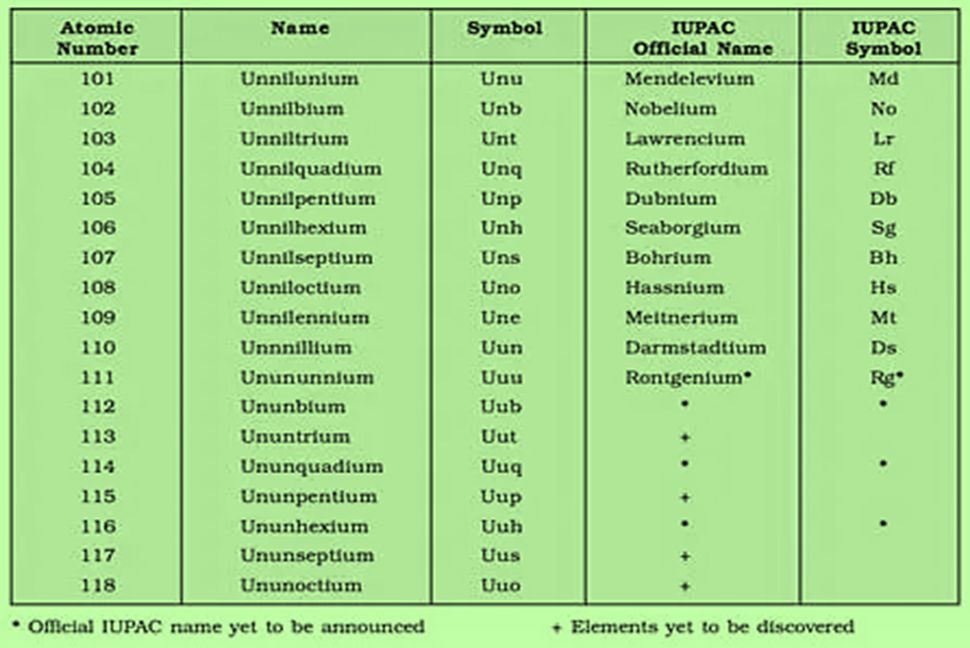
NOMENCLATURE OF ELEMENTS WITH ATOMIC NO. MORE THAN 100
There are around 118 elements in the modern periodic table.Out of 118 elements that have been discovered by scientists, 24 are synthetically developed by mankind and the rest are naturally occurring elements. Few of the elements from the 24 man-made elements were discovered much before and few others were discovered recently by a team that was headed by Glen Seaborg at the Lawrence Berkeley Laboratory in Berkeley, California.
The names and the symbols that are given to these elements are still not used universally. Some of them even had two names and symbols. For example, the element with the atomic number 104 was discovered by America as well as the Soviet Union. The American Scientist gave it the name Rutherfordium (Rf) whereas the name Kurchatovium (Ku) was established by scientists of the Soviet Union. In a similar manner, another element with the atomic number 107 is named Neilsbohrium (Ns) as well as Bohrium (Bh).
Thus a committee named commission on nomenclature of inorganic chemistry (CNIC) was issued by the IUPAC so that a particular IUPAC naming process should be assigned for the elements whose atomic number is greater than or equal to 100. In the year 1997, after an elaborate discussion with all the scientists around the world, IUPAC decided on the official names for elements with atomic numbers 104 to 110 and proposed a method for naming the elements.
In this article, you will get to know about the nomenclature of the elements above 100 and their IUPAC names as well. Generally, the discoverer of the element is given the honour to name the element discovered. The name of the chemical element comes from the physical or chemical properties, its origin, or mythical characters. The recommended name of an element is then consented to by the IUPAC (International Union of Pure and Applied Chemistry).
How The Elements With The Atomic Number (Z) Above 100 Assigned Names?
Most of the chemical elements have been assigned their symbols and names but still are not used universally. While studying the nomenclature of elements with an atomic number greater than 100, it was found that some elements are given two symbols or names.
For instance, an element with atomic number=104 was claimed by both Soviet and American scientists. The Americans assigned the element with the name Rutherfordium (Rf) whereas the Soviets named it Kurchatovium (Ku). Similarly, the element with Z=107 is assigned with the name Bohrium (Bh)
In 1978, IUPAC made a CNIC (Commission on Nomenclature of Inorganic Chemistry) to eradicate the element naming and symbol issues. The IUPAC gives a clear rule of the systematic nomenclature of elements with an atomic number above 100.
The Commission claimed that the elements must be named methodically by following the given below rules:
The names of the elements should be short. Additionally, the element name should be related to its atomic number.
Whether an element of atomic number 100 or above or it is a metal or non-metal, its name should end in ‘ium’.
In the systematic nomenclature of elements with atomic number 100, the element symbols should consist of three letters.
The symbol should be derived from the atomic numbers of the element and be visually connected to the names.
The elements with the atomic numbers (Z) from 101 to 103 have trivial names. Additionally, they have consistent two-letter symbols accepted by IUPAC. The chemical element with an atomic number above 100 is also referred to as super heavy elements.
The Commission mentions the use of three-letter structures for the chemical elements with Z=100 or above it. Because any scientifically derived set of two-letter symbols will tend to duplicate some of the elements of an atomic number less than 104.
Nomenclature Of Elements With Atomic Number (Z) Greater Than 100
The name of the element is directly derived from its atomic number by using the following numerical roots:
0 = nil (n)
1 = un (u)
2 = bi (b)
3 = tri (t)
4 = quad (q)
5 = pent (p)
6 = hex (h)
7 = sept (s)
8 = oct (o)
9 = enn (e)
The numerical roots are put together according to the digits, which make up the atomic number and end with “ium” to spell out the name.
The element symbol consists of the initial letters of the numerical roots, which make up the name.
In the name of the element, each root is pronounced separately such that the root ‘un’ should get pronounced with a long ‘u’.
For example, let us find the name of an element with atomic number 120:
Code for 1 is un
Code for 2 is bi
Code for 0 is nil
Hence the name of the element with atomic number 120 is un + bi + nil + ium (ending code), that is; unbinilium.
However, it is necessary to note that if the last digit code like bi ends with the letter ‘i’ then just add ‘um’. So, the ending, in that case, will be ‘um’ instead of ‘ium’.
5. Electronic configurations
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ELECTRONIC CONFIGURATIONS AND THE PERIODIC TABLE
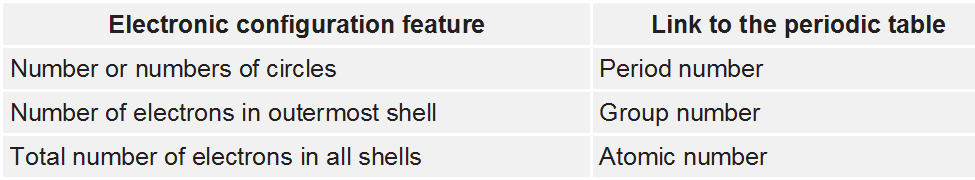
The electronic configuration of an element is related to its position on the periodic table.
the number of circles in the electronic configuration of an element is represented in the periodic table as the period number that element is situated in
the number of electrons in the outermost shell of an element is represented in the periodic table as the group number that element is situated in
the number of electrons in all shells of an element is represented in the periodic table as the element's atomic number
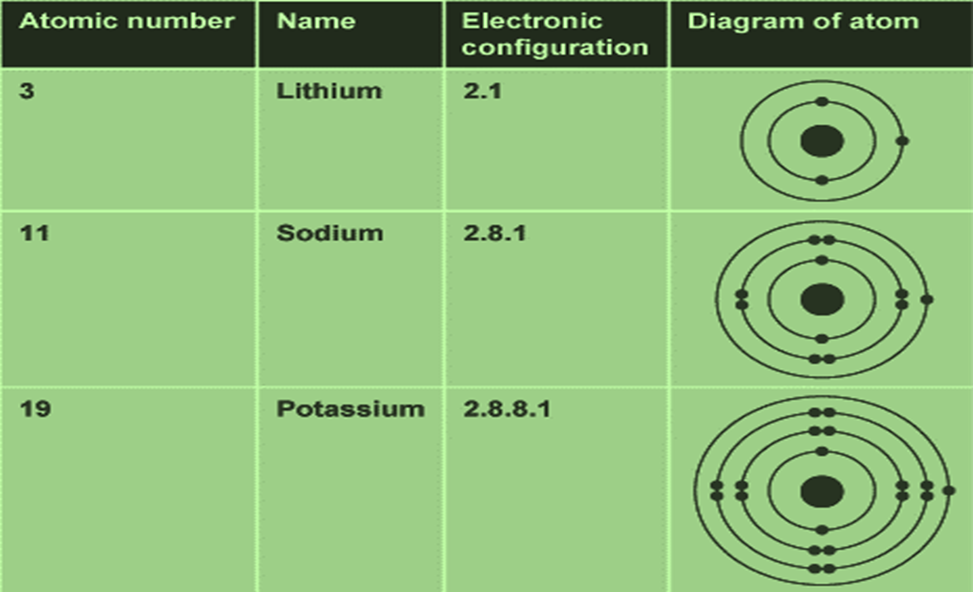
The electronic configuration of sodium shows that sodium, Na:
- is in period 3
- is in group 1
- has an atomic number of (2 + 8 + 1) = 11
Electronic configurations and properties of elements
The electronic configurations of atoms help explain the properties of elements and the structure of the periodic table. When atoms collide and react, it is the outer electrons that meet and interact. So, elements in the same group have similar chemical properties because they have the same number of electrons in their outer shell.
The electronic configurations of the elements in group 1:
The atoms of all group 1 elements have similar chemical properties and reactions because they all have one electron in their outer shell. Similarly, the atoms of all group 7 elements have similar chemical properties and reactions to each other because all of them have seven electrons in their outer shell.
Groups
The long form of periodic table also consists of the vertical rows called groups. There are in all 18 groups in the periodic table. Unlike Mendeleev periodic table, each group is an independent group.
Characteristics of groups:
(i) All the elements present in a group have same general electronic configuration of the atoms.
(ii) The elements in a group are separated by definite gaps of atomic numbers (2, 8, 8,18, 18,32).
(iii) The atomic sizes of the elements in group increase down the group due to increase the number of shells.
(iv) The physical properties of the elements such as m.p., b.p. density, solubility etc., follow a systematic pattern.
(v) The elements in each group have generally similar chemical properties.
6. Electronic configurations and types of elements
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
ELECTRONIC CONFIGURATIONS AND TYPES OF ELEMENTS: s-, p-, d-, f- BLOCKS
Periods
Horizontal rows in a periodic table are known as periods.
There are in all seven periods in the long form of periodic table.
Characteristics of periods:
(i) In all the elements present in a period, the electrons are filled in the same valence shell.
(ii) The atomic sizes generally decrease from left to right.
s-Block Elements
General electronic configuration: ns1-2 Characteristics of s-block elements:
(i) All the elements are soft metals.
(ii) They have low melting and boiling points.
(iii) They are highly reactive.
(iv) Most of them impart colours to the flame.
(v) They generally form ionic compounds.
(vi) They are good conductors of heat and electricity. p-Block Elements
General electronic configuration: ns2np1-6
Characteristics of p-block elements:
(i) The compounds of these elements are mostly covalent in nature.
(ii) They show variable oxidation states.
(iii) In moving from left to right in a period, the non-metallic character of the elements increases.
(iv) The reactivity of elements in a group generally decreases downwards.
(v) At the end of each period is a noble gas element with a closed valence shell ns2 np6 configuration.
(vi) Metallic character increases as we go down the group.
d-Block Elements:
General electronic configuration: (n -1) d1-10 ns0-2
The d-block elements are known as transition elements because they have incompletely filled d-orbitals in their ground state or in any of the oxidation states.
Characteristics of d-block elements:
(i) They are all metals with high melting and boiling points.
(ii) The compounds of the elements are generally paramagnetic in nature.
(iii) They mostly form coloured ions, exhibit variable valence (oxidation states).
(iv) They are of tenly used as catalysts.
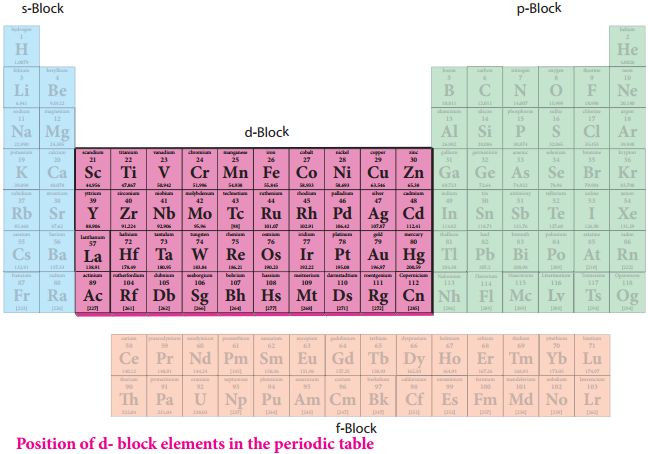
f-Block Elements
General electronic configuration: (n – 2) f1-14 (n -1) d0-1 ns2
They are known as inner transition elements because in the transition elements of d-block, the electrons are filled in (n – 1) d sub-shell while in the inner transition elements of f-block the filling of electrons takes place in (n – 2) f subshell, which happens to be one inner subshell.
Characteristics of f-Block elements:
(i) The two rows of elements at the bottom of the Periodic Table, called the Lanthanoids Ce (Z = 58) – Lu (Z = 71) and Actinoids Th (Z = 90) – Lr (Z = 103).
(ii) These two series of elements are called Inner Transition Elements (f-Block Elements).
(iii) They are all metals. Within each series, the properties of the elements are quite similar.
(iv) Most of the elements pf the actinoid series are radio-active in nature.
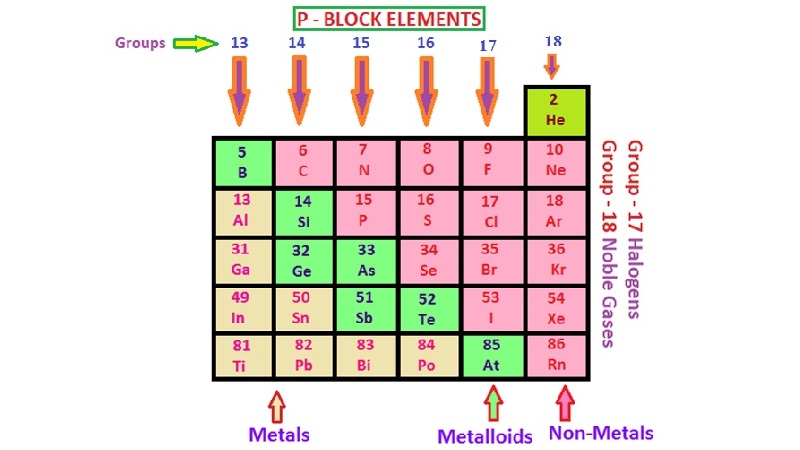
• Metals
(i) Metals comprise more than 78% of all known elements and appear on the left side of the Periodic Table.
(ii) Metals are solids at room temperature.
(iii) Metal usually have high melting and boiling points.
(iv) They are good conductors of heat and electricity.
(u) They are malleable and ductile.
• Non-metals
(i) Non-metals are located at the top right hand side of the Periodic Table.
(ii) Non-metals are usually solids or gases at low temperature with low melting and boiling points.
(iii) They are poor conductors of heat and electricity.
(iv) The non-metallic character increases as one goes from left to right across the Periodic
Table.
(v) Most non-metallic solids are brittle and are neither malleable nor ductile.
• Metalloids
The elements (e.g., silicon, germanium, arsenic, antimony and tellurium) show the characteristic, of both metals and non-metals. These elements are also called semimetal.
• Noble Gases
– These are the elements present in group 18.
– Eash period ends with noble gas element.
– All the members are of gaseous nature and because of the presence of all the occupied filled orbitals, they have very little tendency to take part in chemical combination.
– These are also called inert gases.
• Representative Elements
The elements of group 1 (alkali metals), group 2 (alkaline earth metals) and group 13 to 17 constitute the representative elements. They are elements of s-block and p-block.
• Transition Elements
The transition elements include, all the d-block elements and they are present in the centre of the periodic table between s and p-block elements.
• Inner Transition Elements
Lanthanoids (the fourteen elements after Lanthanum) and actinides (the fourteen elements after actinium) are called inner transition elements. They are also called f-block elements.
The elements after uranium are also called transuranic elements.
7. Periodic trends of elements
- Books Name
- Ritan Sheth Chemistry Book
- Publication
- Ritan Sheth
- Course
- CBSE Class 11
- Subject
- Chemistry
PERIODIC TRENDS IN PROPERTIES OF ELEMENTS
Trends in Physical Properties
Atomic Radii: It is defined as the distance from the centre of the nucleus to the outermost shell containing the electrons. Depending upon whether an element is a non-metal or a metal, three different types of atomic radii are used. These are:
(a) Covalent radius (b) Ionic Radius (c) van der Waals radius (d) Metallic radius.
(a) Covalent Radius: It is equal to half of the distance between the centres of the nuclei of two atoms held together by a purely covalent single bond.
(b) Ionic Radius: It may be defined as the effictive distance from the nucleus of an ion upto which it has an influence in the ionic bond.
(c) van der Waals Radius: Atoms of Noble gases are held together by weak van der Waals forces of attraction. The van der Waals radius is half of the distance between the centre of nuclei of atoms of noble gases.
(d) Metallic Radius: It is defined as half of the intemuclear distance between the two adjacent metal ions in the metallic lattice.
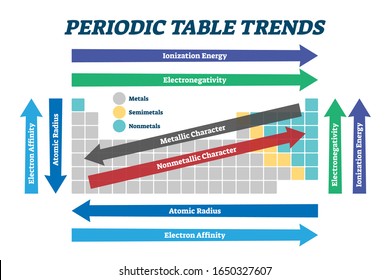
• Variation of Atomic Radius in the Periodic Table
Variation in a Period: Along a period, the atomic radii of the elements generally decreases from left to right.
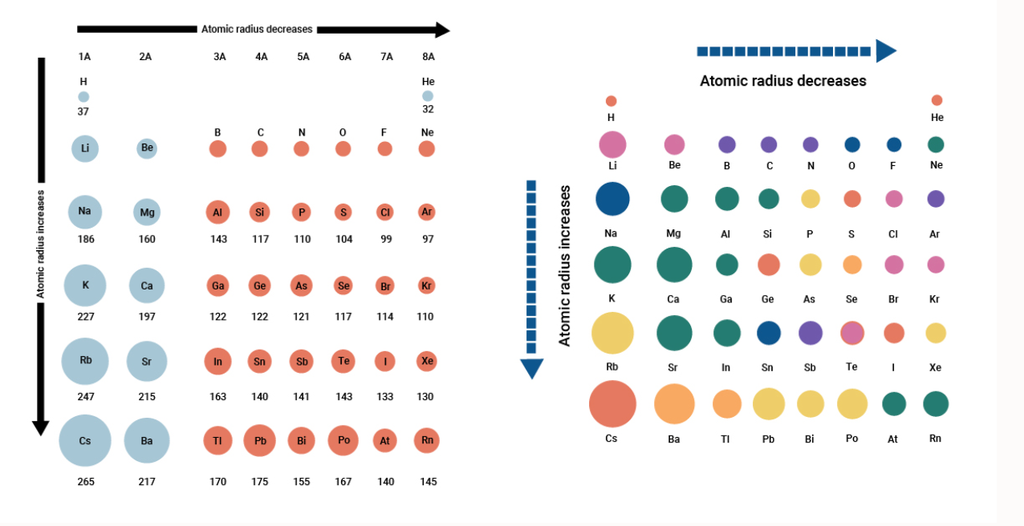
Variation in a group: The atomic radii of the elements in every group of the periodic table increases as we move downwards.
• Ionic Radius
The ionic radii can be estimated by measuring the distances between cations and anions in ionic crystals.
In general, the ionic radii of elements exhibit the same trend as the atomic radii.
Cation: The removal of an electron from an atom results in the formation of a cation. The radius of cation is always smaller than that of the atom.
Anion: Gain of an electron leads to an anion. The radius of the anion is always larger than that ‘ of the atom.
Isoelectronic Species: Some atoms and ions which contain the same number of electrons, we call them isoelectronic species. For example, O2-, F–, Na+ and Mg2+ have the same number of electrons (10). Their radii would be different because of their different nuclear charges.
• Ionization Enthalpy
It is the energy required to remove an electron from an isolated gaseous atom in its ground state.
M (g) + I.E ——->M+ (g) + e–
The unit of ionization enthalpy is kJ mol-1 and the unit of ionization potential is electron volt per atom.
Successive Ionization Enthalpies
If a gaseous atom is to lose more than one electron, they can be removed one after the other i.e., in succession and not simultaneously. This is known as successive ionization enthalpy (or potential).
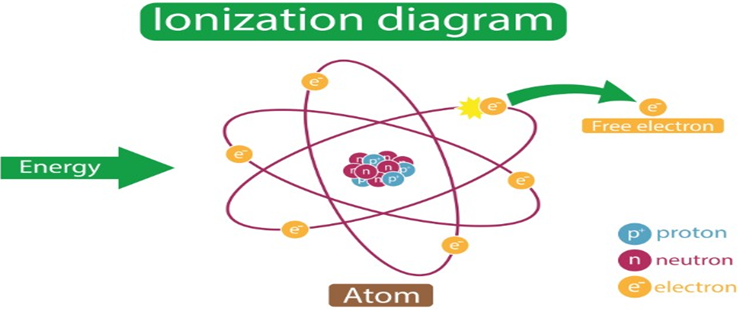
• Variation of Ionization Enthalpies in the Periodic Table:
Variation of Ionization Enthalpy Along a Period
Along a period ionization enthalpies are expected to increase in moving across from left to the right, because the nuclear charge increases and the atomic size decreases.
Variation of Ionization Ethalpy in a Group
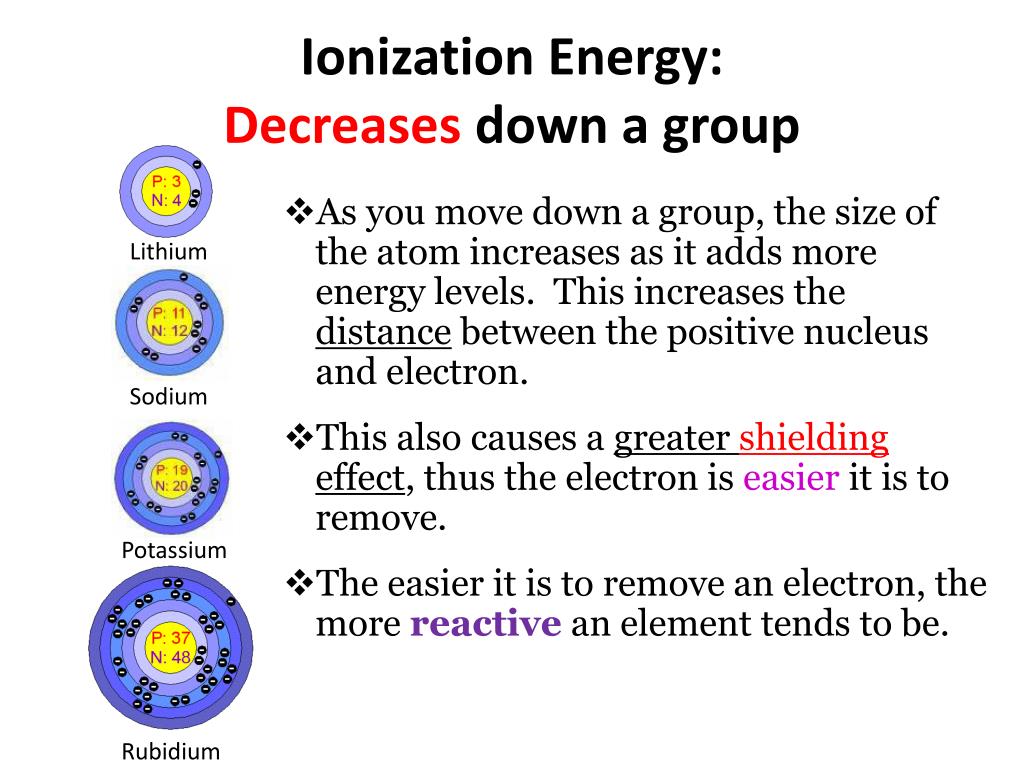
The ionization enthalpies of the elements decrease on moving from top to the bottom in any group.
The decrease in ionization enthalpies down any group is because of the following factors.
(i) There is an increase in the number of the main energy shells (n) in moving from one element to the other.
(ii) There is also an increase in the magnitude of the screening effect due to the gradual increase in the number of inner electrons.
M(g) IE1 M+ + e-
M+(g) IE2 M2+ + e-
M2+(g) IE3 M3+ + e-
• Electron Gain Enthalpy
Electron Gain Enthalpy is the energy released when an electron is added to an isolated gaseous atom so as to convert it into a negative ion. The process is represented as:
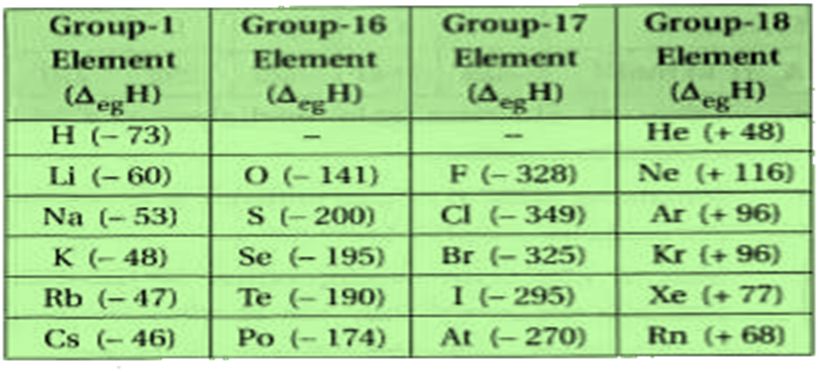
For majority of the elements the electron gain enthalpy is negative. For example, the electron gain enthalpy for halogens is highly negative because they can acquire the nearest noble gas configuration by accepting an extra electron.
In contrast, noble gases have large positive electron gain enthalpies because the extra electron has to be placed in the next higher principal quantum energy level thereby producing highly unstable electronic configuration.
Successive Electron Gain Enthalpies
We have studied that electrons from a gaseous atoms are lost in succession (i.e., one after the other). Similarly, these are also accepted one after the other, i.e., in succession. After the addition of one electron, the atom becomes negatively charged and the second electron is to be added to a negatively charged ion. But the addition of second electron is opposed by electrostatic repulsion and hence the energy has to be supplied for the addition of second electron. Thus the second electron gain enthalpy of an element is positive.
For example, when an electron is added to oxygen atom to form O– ion, energy is released. But when another electron is added to 0- ion to form O2- ion, energy is absorbed to overcome the strong electrostatic repulsion between the negatively charged 0– ion and the second electron being added. Thus, first electron gain enthalpy:
X(g) + e- X- (g)
Factors on which Electron Gain Enthalpy Depends
(i) Atomic size: As the size of an atom increases, the distance between its nucleus and the incoming electron also increases and electron gain enthalpy becomes less negative,
(ii)Nuclear charge: With the increase in nuclear charge, force of attraction between the nucleus and the incoming electron increases and thus electron gain enthalpy becomes more negative.
(iii) Symmetry of the Electronic Configuration: The atoms with symmetrical configuration (having fully filled or half filled orbitals in the same sub-shell) do not have any urge to take up extra electrons because their configuration will become unstable.
In that case the energy will be needed and electron gain enthalpy (Δ eg H) will be positive. For example, noble gas elements have positive electron gain enthalpies.
Variation of Electron Gain Enthalpy Across a Period
Electron gain ethalpy becomes more negative with increase in the atomic number across a period.
Variation of Electron Gain Enthalpy in a Group
Electron gain enthalpy becomes less negative as we go down a group.
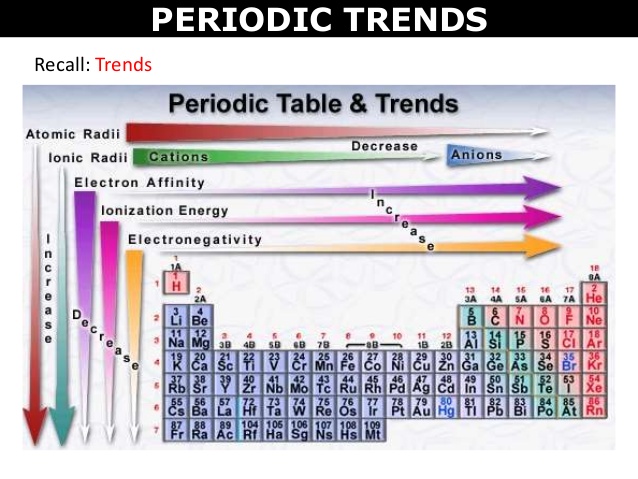
• Electronegativity
A qualitative measure of the ability of an atom in a chemical compound to attract shared electrons to itself is called electronegativity. Unlike ionization enthalpy and electron gain enthalpy, it is not a measurable quantity.
However, a number of numerical scales of electronegativity of elements viz, Pauling scale, Milliken- Jaffe scale, Allred Kochow scale have been developed. The electronegativity of any given element is not constant; it varies depending on the element to which it is bound.
Across a Period
Electronegativity generally increases across a period from left to right.
In a Group
It decreases down a group.
• Periodic Trends in Chemical Properties along a Period
(i) Metallic character: Decrease across a period maximum on the extreme left (alkali metals).
(ii) Non-metallic character: Increasess along a period. (From left to right).
(iii) Basic nature of oxides: Decreases from left to right in a period.
(iv) Acidic nature of oxides: Increases from left to right in a period.
• Variation from Top to Bottom on Moving Down a Group
(i) Metallic character. Generally increases because increase in atomic size and hence decrease in the ionizatiort energy of the elements in a group from top to bottom.
(ii) Non-metallic character. Generally decreases down a group. As electronegativity of elements decreases from top to bottom in a group.
(iii) Basic nature of oxides. Since metallic character or electropositivity of elements increases in going from top to bottom in a group basic nature of oxidise naturally increases.
(iv) Acidic character of oxides. Generally decreases as non-metallic character of elements decreases in going from top to bottom in a group.
(v) Reactivity of metals. Generally increases down a group. Since tendency to lose electron increases.
(vi) Reactivity of non-metals. Generally decreases down the group, Higher the electro-negativity of non-metals, greater is their reactivity. Since electronegativity of non-metals in a group decreases from top to bottom, their reactivity also decreases.
• Anomalous Properties of Second Period Elements
The first element of each of the group 1 (lithium) and 2 (beryllium) and group 13-17 (boron to fluorine) differs in many respect from the other members of their respective groups. For example, lithium unlike other alkali metals, and beryllium unlike other alkaline earth metals
form compounds which have significant covalent character; the other members of these groups, pre-dominatly form ionic compounds.
It has been observed that some elements of the second period show similarities with the elements of the third period placed diagonally to each other, though belonging to different groups.
For example,
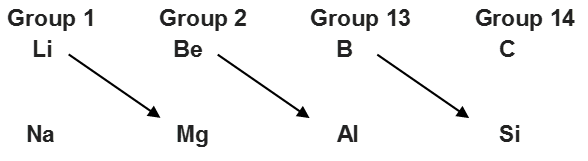
This similarity in properties of elements placed diagonally to each other is called diagonal relationship.
• Mendeleev’s Periodic Law. Physical and chemical properties of elements are periodic function of their atomic masses.
• Modem Periodic Law. Physical and chemical properties of the elements are periodic function of their atomic numbers.
• Groups. There are 18 groups. These are vertical rows.
• Periods. There are 7 periods. These are horizontal rows.
• Representative Elements. The S and P block of elements are known as representative
elements.
• Transition Elements. They are also called d-block elements. They have general electronic
configuration (n – 1) d1-10 ns0-2.
• Inner Transition Elements. Lanthanoids (the fourteen elements after Lanthanum) and actinides (the fourteen elements after actinium) are called inner transition elements. General electronic configuration is (n – 2) f1-14(n – 1) d0-1 ns2.
They are also called f-block elements.
• Metals. Present on the left side of the periodic table. Comprise more than 78% of the known elements.
• Non-metals. Mostly located on the right hand side of the periodic table.
• Metalloids. Elements which line as the border line between metals and non-metals (e.g., Si, Ge, As) are called metalloids or semimetals.
• Atomic Radii and Ionic Radii, increase down the group decrease along the period.
• Ionization Enthalpy. Increases along the period and decreases down the group.
• Noble Gas Elements. Elements with symmetrical configuration are chemically inert in nature.
• Electric Nuclear Charge. Z = Nuclear charge – Screening constant.
• Electronegativity. Increases along a period decreases down the group,
• Chemical Reactivity. Chemical reactivity is highest at the two extremess of a period and lowest
in the centre.
• Oxides of Elements. Oxides formed of the Elements on the left are basic and of elements
on the right are acidic in nature.
Oxides of elements in the centre are amphoteric or neutral.

 Ritan Sheth
Ritan Sheth
