INTRODUCTION
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Introduction
→ Around 500 B.C., Indian philosopher Maharishi Kanad, postulated the theory if we go on dividing
matter (padarth), we will obtain smallest particle beyond which further division can't be possibile
which is known as 'parmanu'.
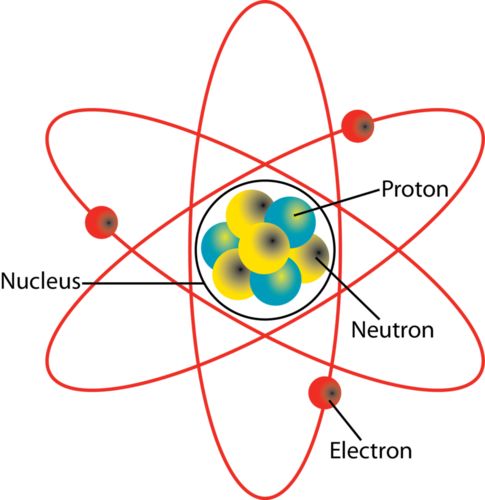
→ Ancient Greek philosophers – Democritus and Leucippus called these particles atoms.
→ Antoine L. Lavoisier laid the foundation of chemical sciences by establishing two important laws
of chemical combination.
1. Dalton's atomic Theory
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Introduction
→ Around 500 B.C., Indian philosopher Maharishi Kanad, postulated the theory if we go on dividing
matter (padarth), we will obtain smallest particle beyond which further division can't be possibile
which is known as 'parmanu'.
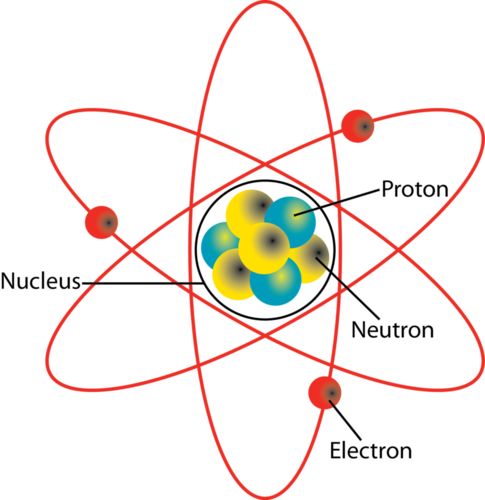
→ Ancient Greek philosophers – Democritus and Leucippus called these particles atoms.
→ Antoine L. Lavoisier laid the foundation of chemical sciences by establishing two important laws
of chemical combination.
Laws of Chemical Combination
• This law established after the experiments by Lavoisier and Joseph L. Proust.
• The chemical reaction between two or more substances give rise to products which is governed
by certain laws called Laws of Chemical Combination.
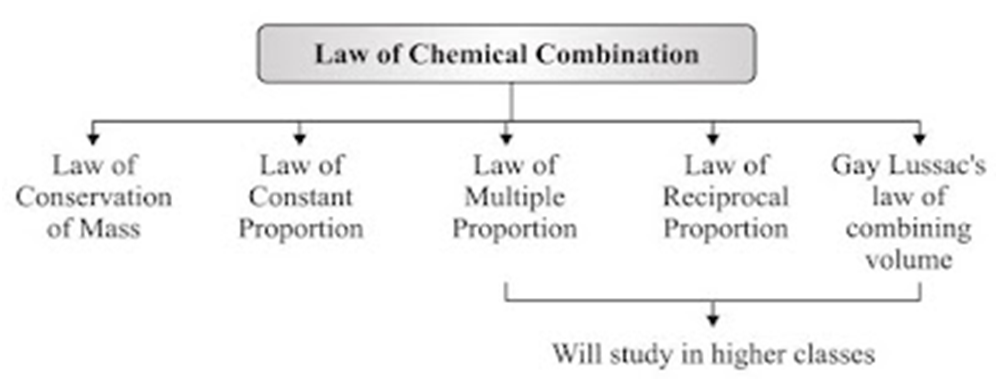
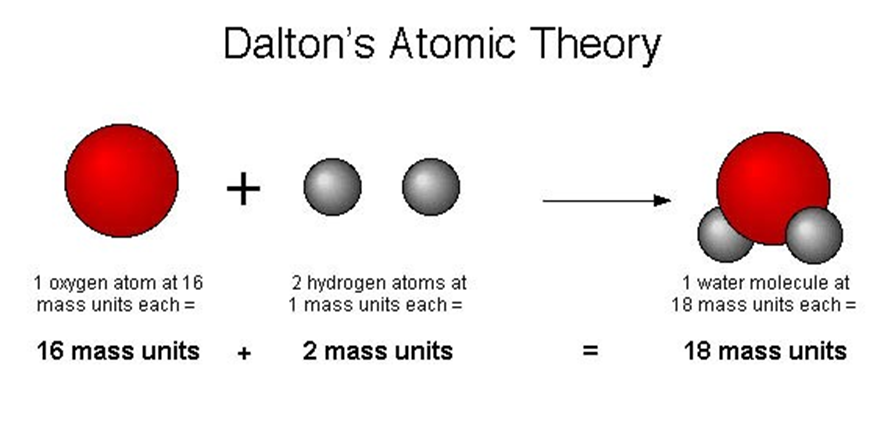
• Law of Conservation of Mass
→ During a chemical reaction, the total mass of reactants will be equal to the total mass of the products.
→ Mass can neither be created nor destroyed in a chemical reaction.
→ Example: A (reactant) + B (reactant) → AB (product)
mass of A + mass of B = mass of AB
• Law of Constant Proportions
→ In a chemical reaction, compounds always contain the same elements present in definite proportions by mass irrespective of their source.
→ It was given by Lavoisier.
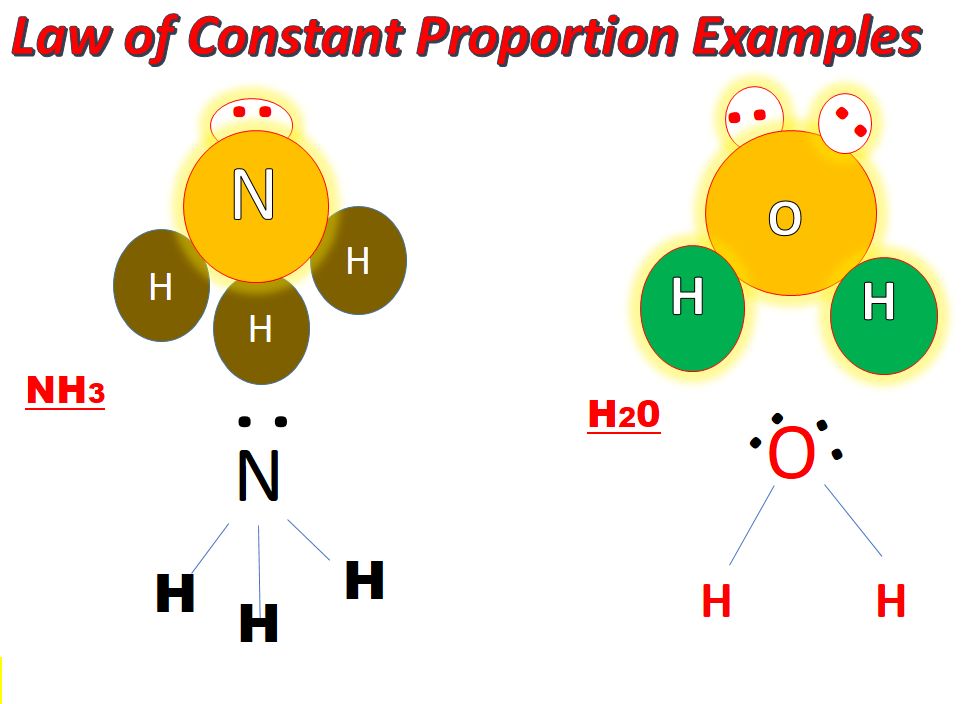
→ For example:
(i) 18 gm of H2O = 2 gm of hydrogen + 16 gm of oxygen
⇒ mass of hydrogen/mass of oxygen = 2/16 = 1/8
(ii) 36 gm of H2O = 4 gm of hydrogen + 32 gm of oxygen
⇒ mass of hydrogen/mass of oxygen = 4/32 = 1/8
(iii) 9 gm of H2O = 1 gm of hydrogen + 8 gm of oxygen
⇒ mass of hydrogen/mass of oxygen = 1/8
This verifies law of constant proportions as the ratio of mass of hydrogen to oxygen is always same.
1. Dalton's atomic Theory
Chapter 3
Atoms & Molecules
Dalton’s Atomic Theory
The matter is made up of indivisible particles known as atoms.
The properties of all the atoms of a given element are the same, including mass. This can also be stated as all the atoms of an element have identical mass and chemical properties; atoms of different elements have different masses and chemical properties.
Atoms of different elements combine in fixed ratios to form compounds.
Atoms are neither created nor destroyed. The formation of new products (compounds) results from the rearrangement of existing atoms (reactants) in a chemical reaction.
The relative number and kinds of atoms are constant in a given compound.
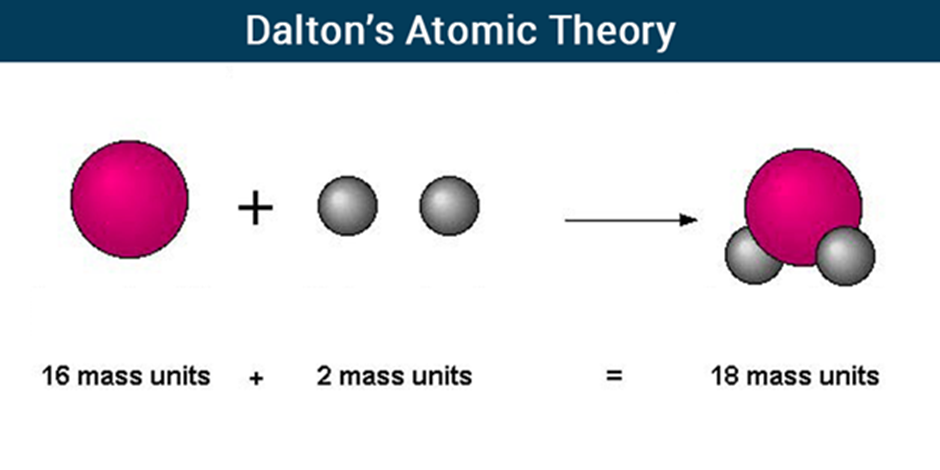
Laws of Chemical Combination
Given by Lavoisier and Joseph L. Proust as follows:
Law of conservation of mass
According to the law of conservation of mass, matter can neither be created nor destroyed in a chemical reaction. It remains conserved.
Mass of reactants will be equal to the mass of products.
Law of constant proportions
A pure chemical compound contains the same elements combined together in a fixed proportion by mass is given by the law of definite proportions.
For e.g., If we take water from a river or from an ocean, both have oxygen and hydrogen in the same proportion.
Atom
Atoms are the smallest particles of an element which can take part in a chemical reaction.
Size of an atom: atomic radius is measured in nanometres.
The atomic symbol has three parts: -
The symbol X: the usual element symbol
The atomic number A: equal to the number of protons
The mass number Z: equal to the total number of protons and neutrons in an element.
1. Dalton's atomic Theory
- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
Chapter-3
Atoms and Molecules
Atoms and Molecules Introduction
We come across different things around us like chair, table, etc. and all the things that surround us have mass and weight. They all constitute matter. Matter is anything that occupies space and has mass. Matter is made up of small particles called atoms.
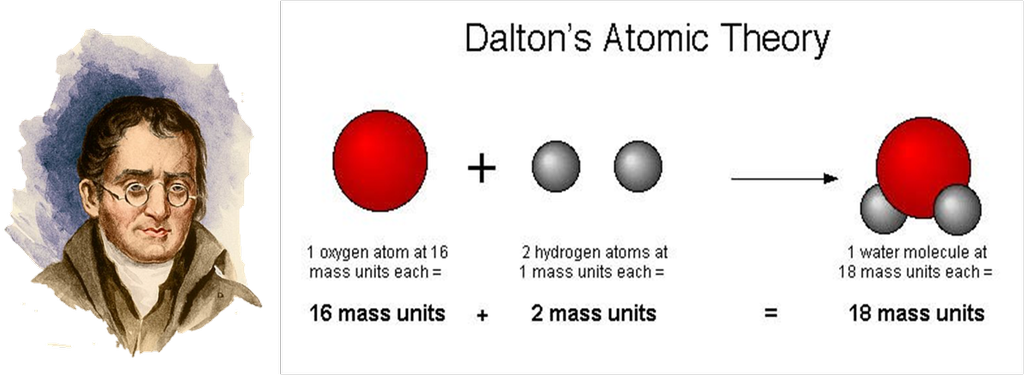
Dalton's atomic Theory
Dalton’s atomic theory
According to Dalton’s Atomic Theory,
- All matter is made up of small particles called atoms.
- Atom is invisible.
- Atom is indivisible.
- Atoms of an element are alike in all aspects.
- Atoms of different elements combine in fixed whole number ratios to form compounds.
Atoms can neither be created nor destroyed.
Drawbacks of Dalton’s Atomic Theory were as follows
1. According to Dalton, an atom is indivisible but later on it was proved that atom can be subdivided into electrons, protons and neutrons.
2. Atoms of an element can somehow differ from each other.
So, these drawbacks led to the failure of Dalton’s theory of an atom. We also know that a lot of chemical reactions take place in our day to day life like making of tea, changing milk to curd, making cheese from milk and lots more. All the chemical reactions taking place obey certain set of laws. Let us study these chemical reactions and the rules that they obey.
Chemical reaction
The process by which some substances react to form a new substance. The substances which react are called reactants and the new ones formed are called products.
For example: A+B C+D
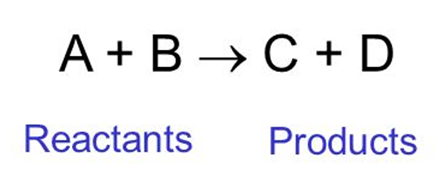
In this, A and B are reactants and C and D are called products.
For example: when we make tea, we add sugar, tea, milk and water. We mix them and heat the mixture. The result is that we get a new substance that is tea.
Laws of chemical combination
There are certain sets of laws that are obeyed by all chemical reactions. These laws are given by Lavoisier & Joseph. Let us study them in detail.
Law of conservation of mass
According to this law, ?matter can not be created nor destroyed in a chemical reaction.? That is, it always remains constant.
Like, in all chemical reactions the total mass of products is equal to the total mass of reactants.
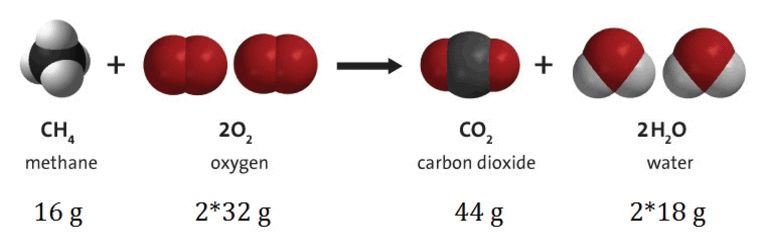
For example:- 2H2 + O2 à 2 H2O
(Reactants) (Product)
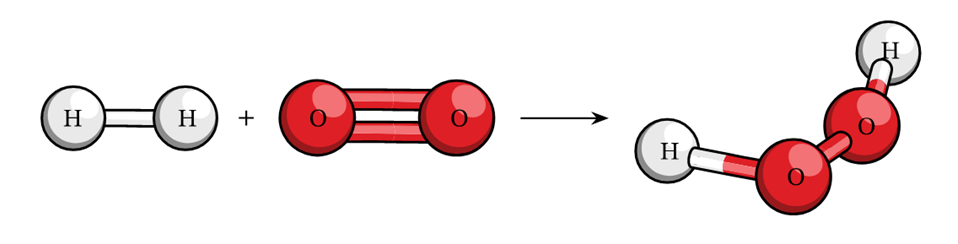
=> 4 + 2 x 16.32 2 x 18 => 36 g = 36 g.
This example shows that the total mass of reactants is equal to the total mass of products formed which is in accordance with the law.
Law of constant proportions: (Law of definite proportion)
According to it, in a chemical substance, the same elements are always present in a definite proportion by mass and volume irrespective of the method of preparation involved. For example, In Co2 molecule, the elements that form it remain the same that is carbon and oxygen and also, the ratio of C and O remains the same that is 3:8 by mass (i.e. 12.32) and 1 : 2 by volume.
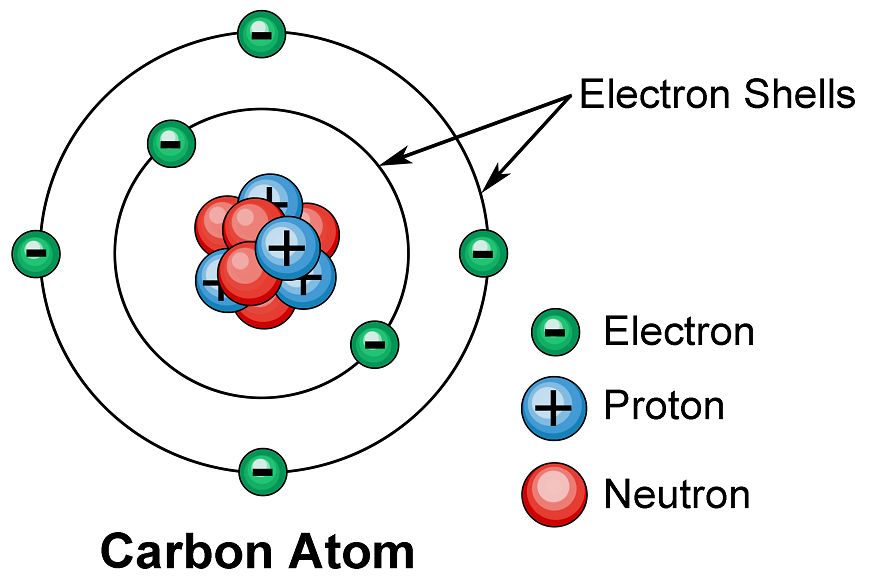
Atom
It is the smallest particle of an element which may as may not have an independent existence. An atom is very small in size. The size is measured in unit of nm (nanometer). 1nm = 10^-9 m. Hydrogen atom is the smallest of all. Its size is only 10-10 nm. Atoms are represented by symbols (given by Berzelius).
2. IUPAC and atomic Symbols
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Dalton's Atomic Theory
→ According to Dalton’s atomic theory, all matter, whether an element, a compound or a mixture is
composed of small particles called atoms.
→ Six Postulates of Dalton's atomic theory:
(i) All matter is made of very tiny particles called atoms.
(ii) Atoms are indivisible particles, which cannot be created or destroyed in a chemical reaction.
(iii) Atoms of a given element are identical in mass and chemical properties. (Law of conservation of mass)
(iv) Atoms of different elements have different masses and chemical properties.
(v) Atoms combine in the ratio of small whole numbers to form compounds. (Law of constant proportion)
(vi) The relative number and kinds of atoms are constant in a given compound.
2. IUPAC and atomic Symbols
Atom
Atoms are the smallest particles of an element which can take part in a chemical reaction.
Size of an atom: atomic radius is measured in nanometres.
The atomic symbol has three parts: -
The symbol X: the usual element symbol
The atomic number A: equal to the number of protons
The mass number Z: equal to the total number of protons and neutrons in an element.
Atomic Mass
Atomic mass and atomic mass unit
Atomic mass is the total of the masses of the electrons, neutrons, and protons in an atom, or in a group of atoms, the average mass.
Mass of an atomic particle is called the atomic mass.
This is commonly expressed as per the international agreement in terms of a unified atomic mass unit (AMU).
It can be best defined as 1/12 of the mass of a carbon -12 atom in its ground state.
Molecule
It is the smallest particle of an element or a compound which can exist independently.
• Molecules of an element constitute the same type of atoms.
• Molecules may be monoatomic, diatomic or polyatomic.
• Molecules of compounds join together in definite proportions and constitute a different type of atoms.
2. IUPAC and atomic Symbols
- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
IUPAC and atomic Symbols
IUPAC and Atomic symbols
IUPAC – International Union Of Pure & Applied Chemistry
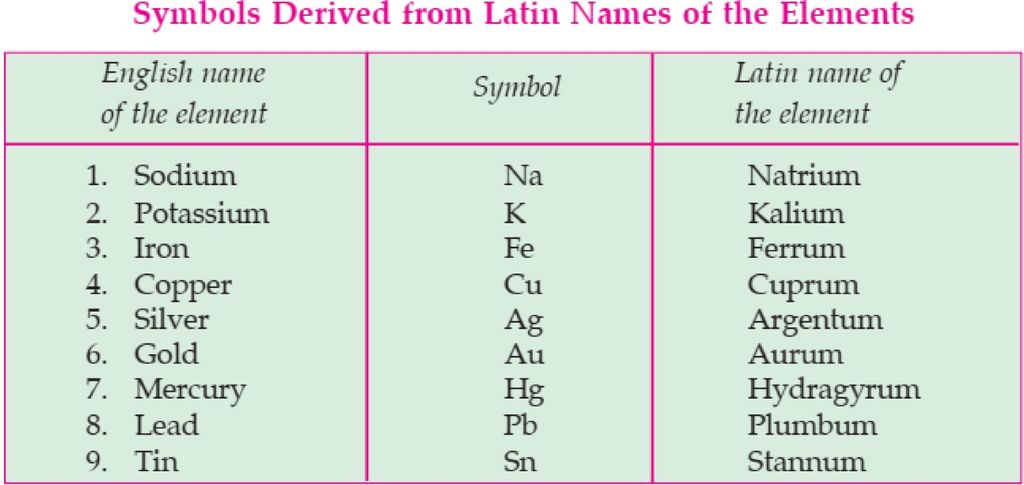
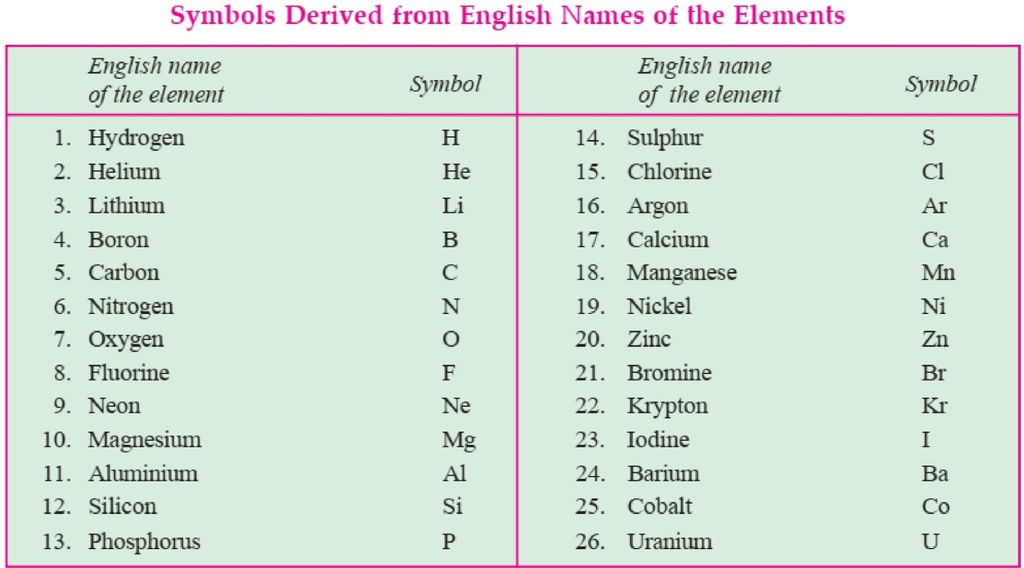
The IUPAC approves the names of elements. According to it,
1. Symbol if an element is either the first letter of the name of the element or the first two letters of the name of an element.
2. In case there are two letters, then the first letter is in a capital case and the second letter is in small case.
For example- lead is written as “Pb” and not as “pb”. Sometimes, the symbol is derived from the Latin name of an element. For example, if we look at the symbol of Sodium- “Na” and not .So, because it is derived from its Latin name that is, “Natrium”. Similarly, for Potassium, Copper, Iron, Mercury, etc. Latin names and symbols of a few elements are given below:
The atomic mass of the element
If we try to find the exact mass of an atom, we can’t find it. The reason being that an atom is too small in size that it is difficult to measure its size and moreover, the size of an atom changes, depending upon its neighbouring- atoms. Therefore, instead of finding its absolute mass, we try to find the relative mass of an atom. The standard element chosen for finding relative mass is carbon-6.
The relative atomic mass of an atom of an element is defined as the average relative mass of an atom as compared to an atom of 612c taken as 12u.
Atomic mass: In simple language, atomic mass is the number of times an atom of an element is heavier than 1/12th of a Carbon atom. For example: If we say that the atomic mass of Sodium is 23, it means that sodium is 23 times heavier than 1/12th of a carbon atom.
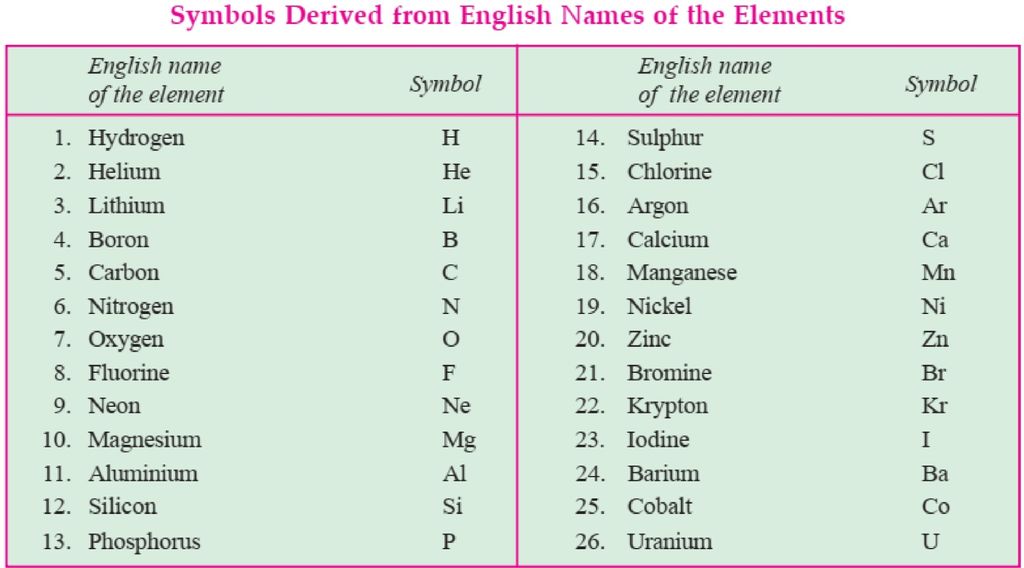
Molecule
The particle next to an atom is a molecule, but it has an independent existence and is formed when atoms combine with each other. Let us study about it. A Molecule is the smallest particle of an element or compound which is able to exist independently. For example, carbon dioxide molecule, hydrogen gas molecule, etc . But it has been seen that some molecules are formed by the combination of two atoms of the same kind like H2 and some may have two or more atoms of different kinds like H2O. This shows that in a molecule any number of atoms can be present and they may be of the same or different kinds.

Atomicity: It is the number or the kinds of atoms present in a molecule of an element. Some more examples: Phosphorous exists as P4 (tetra atomic), Sulphur exists as S8 (polyatomic), Nitrogen exists as N2 (diatomic).
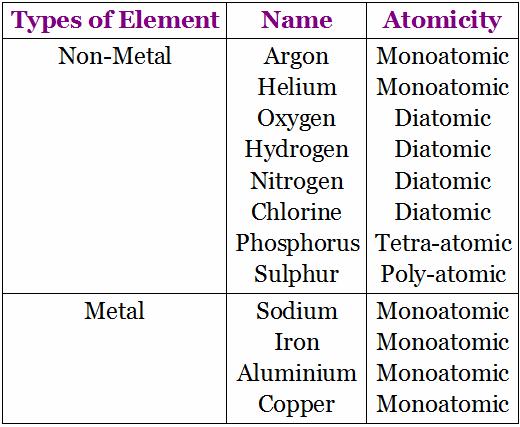
3. Compound
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Atoms
• Atoms are building blocks of all matter.
• According to modern atomic theory, an atom is the smallest particle of an element which taking
part in chemical reaction.
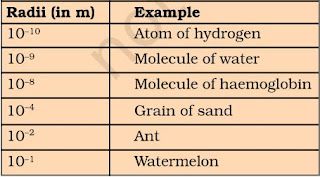
• Atoms are very small and which can’t be seen even through very powerful microscope.
• Atomic radius is measured in nanometres. Nanometres = 10-9 m.
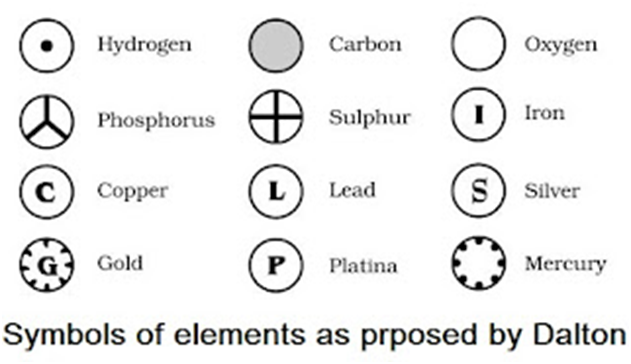
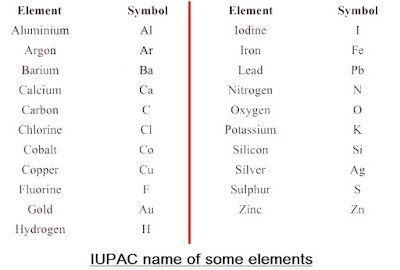
• Modern day symbols of Elements
→ Dalton was the first scientist to use the symbols for elements.
→ Berzilius suggested that the symbols of elements should be made from one or two letters of
name of the element.
→ The name copper was taken from Cyprus, a place from where it was found for first time.
→ Now, IUPAC (International Union of Pure and Applied Chemistry) approves names of element
→ The first letter of a symbol is always written as a capital letter (uppercase) and the second letter.
as a small letter (lowercase). For example: hydrogen (H), aluminium (Al), cobalt (Co).
→ Some other symbols have been taken from the names of elements in Latin, German or Greek.For
example: Fe from its Latin name ferrum, sodium is Na from natrium, potassium is K from kalium.
• Atomic Mass
→ Dalton’s atomic theory proposed the idea of atomic mass which explained the law of constant
proportions so well.
→ The mass of an atom of an element is called its atomic mass.
→ In 1961, IUPAC have accepted ‘atomic mass unit’ (u) to express atomic and molecular mass of
elements and compounds.
→ The atomic mass unit is defined as the quantity of mass equal to 1/12 of mass of an atom of
carbon-12.
1 amu or u = 1/12 × Mass of an atom of C -12
1 u = 1.66 × 10-27 kg

• Atom existence
→ Atoms of most of the elements are very reactive and does not exist in free state.
→ Only the atoms of noble gases (such as He, Ne, Ar, Kr, Xe and Rn) are chemically unreactive and
can exist in the free state as single atom.
→ Atoms of all other elements combine together to form molecules or ions.
3. Compound
Atomicity
The number of atoms constituting a Molecule is known as its atomicity.
Compounds
A pure substance made up of two or more elements chemically combine together in a fixed ratio under fixed condition is called compound. Example: Calcium carbonate, Common salt, Sugar.
Properties of compound
1.Compounds can be separated into the constituent only by chemical methods.
2. Properties of compound differ from the properties of their constituents.
3. During the formation of a compound energy is absorbed or released.
4. The compound are homogeneous.
Valency
The combining capacity of an element is known as its valency. Valency is used to find out how the atom of an element will combine with the atom of another element to form a chemical compound.
(Every atom wants to become stable, to do so it may lose, gain or share electrons.)
• If an atom consists of 1, 2 or 3 electrons in its valence shell then its valency is 1, 2 or 3 respectively,
• If an atom consists of 5, 6 or 7 electrons in the outermost shell, then it will gain 3, 2 or 1 electron respectively and its valency will be 3, 2 or 1 respectively.
• If an atom has 4 electrons in the outermost shell than it will share this electron and hence its valency will be 4.
• If an atom has 8 electrons in the outermost electron and hence its valency will be 0.
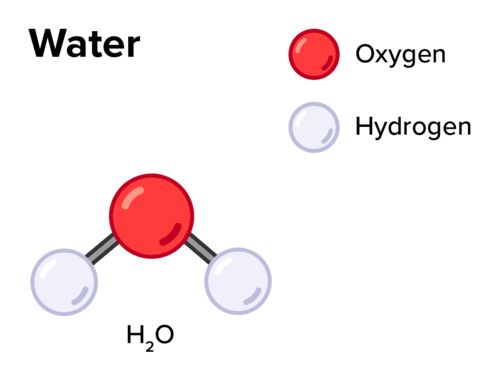
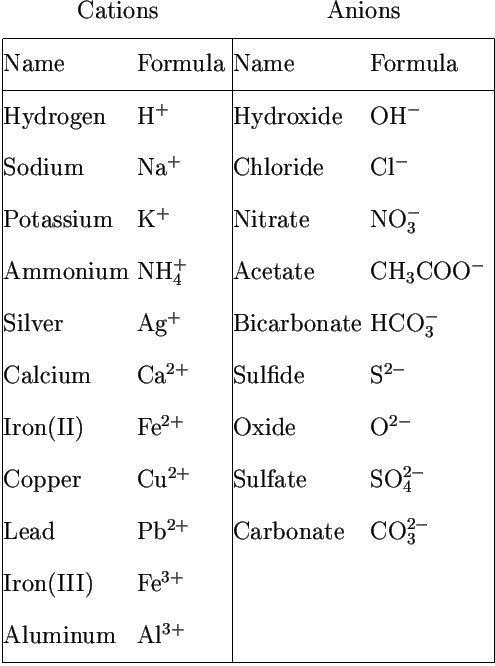
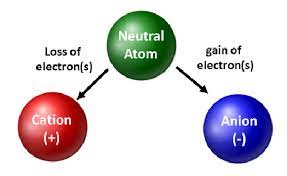
Ions
An ion is an electrically charged atom or group of atoms.
An ion is formed by the loss or gain of electrons by an atom, so it contains an unequal number of electrons and protons.
There are two types of ions:
Cation: A positively charged ion is known as cation.
For Ex: Na+, Mg2+
A cation is formed by the loss of one or more electrons by an atom.
Na – e– ——–>Na+
Z=11 Z=10
2,8,1 2,8
K, L, M K, L
Mg – e– ——–> Mg2+
Z=12 Z=10
2,8,2 2,8
K,L,M K,L
Anion: A negatively charged ion is known as anion.
For Ex: Cl–, O2-
An anion is formed by gain of one or more electron by an atom.
Cl + e– ——–> Cl–
Z=17 Z=18
2,8,7 2,8,8
K, L, M K, L, M
O + e– ———> 02-
Z=8 Z=10
2,6 2,8
K, L K, L
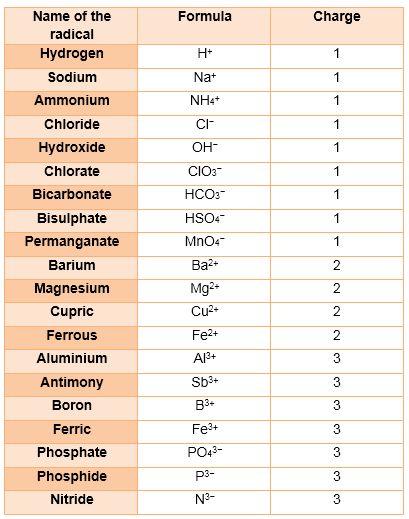
Radicals
An atom or group of atoms having a charge, i. e. either negative or positive, on it.
The radicals having positive charge are called cations.eg. Sodium ion.
The radicals having negative charge are called anions. eg. Chloride ion.
3. Compound
- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
Compound
Compound
When atoms of two or more different elements combine together in a definite proportion by mass and volume, it forms a compound. for example:- H2O is formed only when the ratio of H and O is 2:1 by volume and ratio by mass is 1:8.
Ion
We know that the atom is neutral, that is, the number of positive and negative charges in it is equal but if sometimes, an atom gains or loses negative or positive charge, it no longer remains neutral. It becomes an “ion”. Let us study about it.
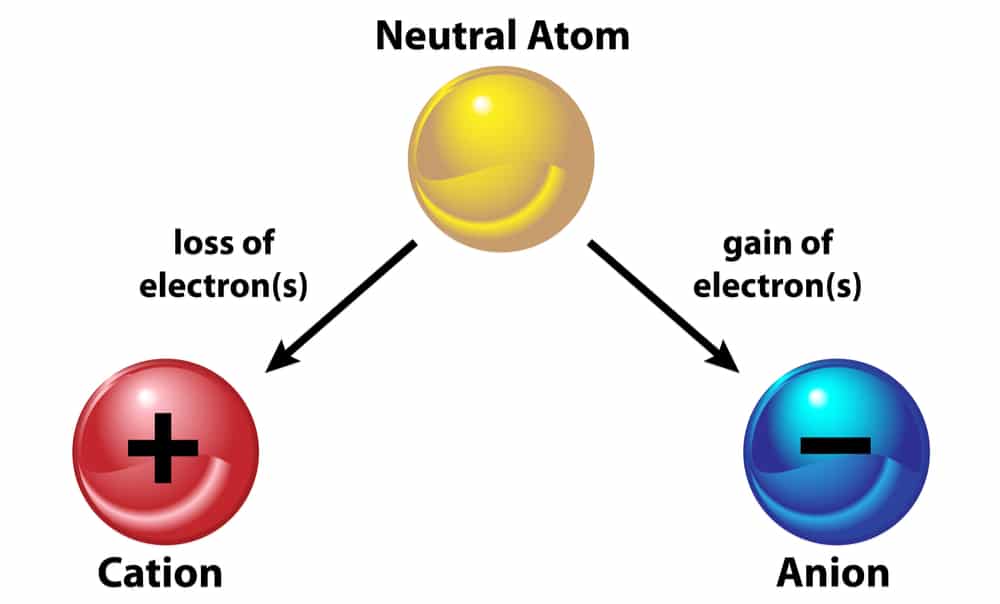
Ion:- The atom that bears a charge is called an “ion”. Ion is of two types:
1. Cation
2. Anion
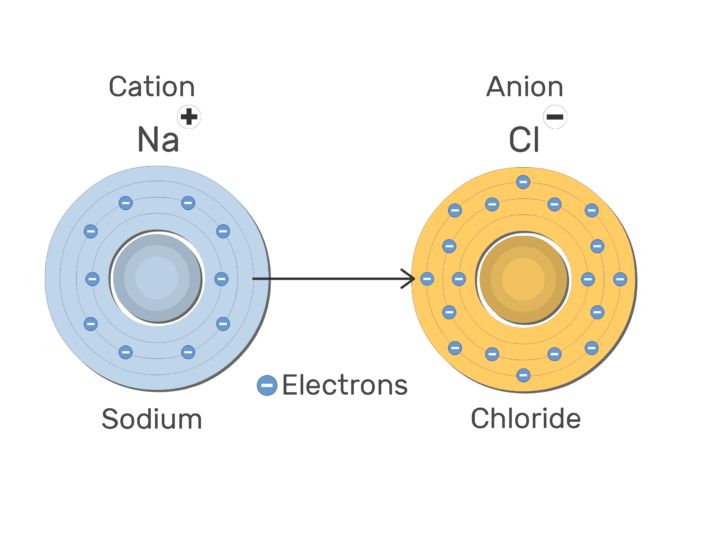
Cation: they are positively charged ions. They are formed when an atom loses a negative charge. Therefore, they always have negative charges less than normal atoms. Example: Na -1 negative charge Na+ (cation)
Anion: they are negatively charged ions and are formed when an atom gains a negative charge. Therefore, they have negative charges more than normal atoms. Example: S +2 negative charges S2- (anion)
>> 7.2. You might expect that by this time in the history of modern civilization science would have ..." style="width:389px; height:523px" data-cke-upload-id="3" data-widget="uploadimage">
Valency and Radicals
The group of atoms that bears a charge is also called a polyatomic ion. The following radicals are given below:
4. Chemical Formula
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Molecules
→ A molecule is in general a group of two or more atoms that are chemically bonded together.
→ A molecule is the smallest particle of matter (except element) which is capable of an independent existence and show all properties of that substance.
→ Examples: ‘H2O’ is the smallest particle of water which shows all the properties of water.
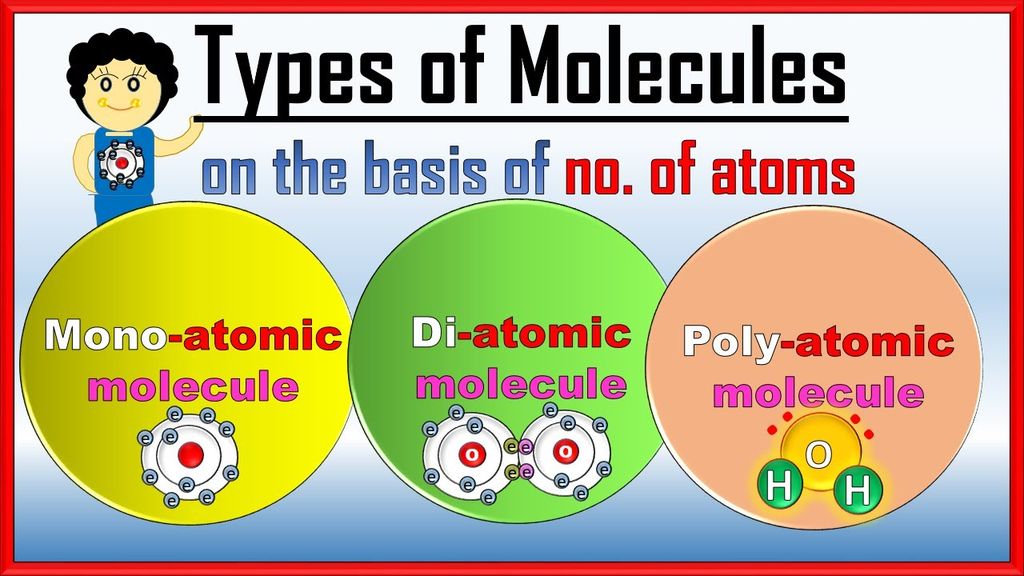
→ A molecule may have atom of same or different elements, depending upon this, molecule can be categorized into two categories:
(i) Homoatomic molecules (containing atom of same element)
Examples: H2, O2, O3 , S8 , P4 etc.
(ii) Heteroatomic molecules or compounds (containing atoms of different elements)
Examples: H2O, CO2 , NaCl, CaCO3 etc.
4. Chemical Formula
Chemical Formulae
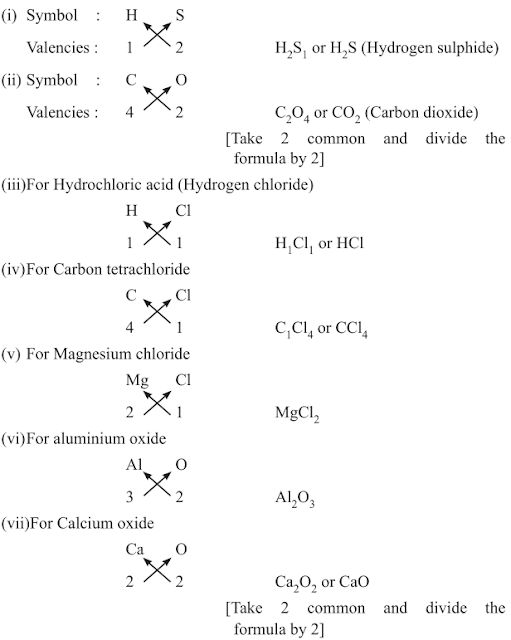
Rules:
(i) The valencies or charges on the ion must balance.
(ii) Metal and non-metal compound should show the name or symbol of the
metal first.
e.g., Na+ Cl– → NaCl
(ii) If a compound consists of polyatomic ions. The ion is enclosed in a bracket before writing the number to indicate the ratio.
e.g., [SO4]2- → polyatomic radical
H1+ SO42- → H2SO4
Molecular Mass
It is the sum of the atomic masses of all the atoms in a molecule of the substance. It is expressed in atomic mass unit (u).
Formula Unit Mass
It is the sum of the atomic masses of all atoms in a formula unit of a compound. The constituent particles are ions.
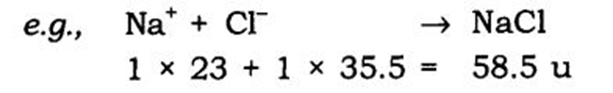
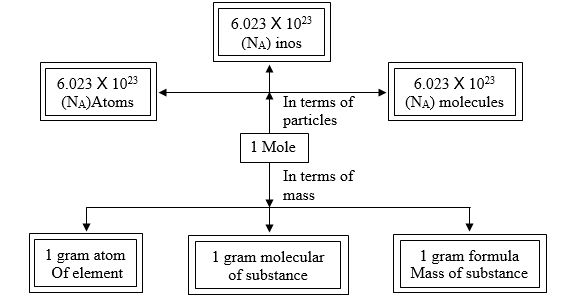
Mole Concept
Definition of mole: It is defined as one mole of any species (atoms, molecules, ions or particles) is that quantity in number having a mass equal to its atomic or molecular mass in grams.
1 mole = 6.022 x 1023 in number
Molar mass = mass of 1 mole → is always expressed in grams and is also known as gram atomic mass.
l u of hydrogen has → 1 atom of hydrogen and 1g of hydrogen has → 1 mole of hydrogen
= 6.022 x 1023 atoms of hydrogen.
4. Chemical Formula
- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
Chemical Formula
Chemical formula
Chemical Formula is the symbolic representation of compound. For example, the compound ammonium nitrate is written as
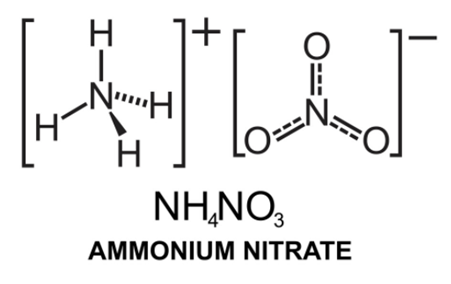
4NO3) - Structure, Preparation, Physical and Chemical Properties, Uses with FAQs of Ammonium Nitrate" style="width:286px; height:179px" data-cke-upload-id="0" data-widget="uploadimage">
If we say that water is represented as H2O, we get the following information from it
1. H2O represents water.
2. H2O represents one molecule of water.
3. It represents that 1molecule of H2O contain 2Hydrogen and 1 Oxygen atoms.
4. It represents 18g of water.
5. It represents that 1 molar mass of water has 6.022 x 10 23 molecules.
Mass percentage
Mass Percentage is the ratio of mass of a substance whose percentage is to be calculated to the total mass of the substance multiplied by 100.
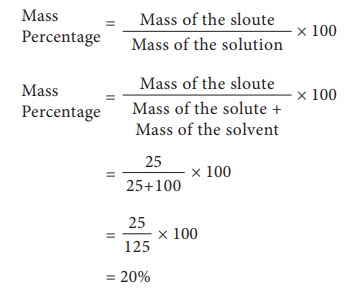
Example 1: Find the mass percentage of 6 g sodium hydroxide dissolved in 50 g of water. (Note: since the density of water is nearly 1, this type of question often gives the volume of water in milliliters.)
First find the total mass of the solution:
total mass = 6 g sodium hydroxide + 50 g water
total mass = 56 g
Now, you can find the mass percentage of the sodium hydroxide using the formula:
mass percent = (grams of solute / grams of solution) x 100
mass percent = (6 g NaOH / 56 g solution) x 100
mass percent = (0.1074) x 100
answer = 10.74% NaOH
Example 2: Find the masses of sodium chloride and water required to obtain 175 g of a 15% solution.
This problem is a bit different because it gives you the mass percentage and asks you to then find how much solute and solvent are needed to yield a total mass of 175 grams. Start with the usual equation and fill in the given information:
mass percent = (grams solute / grams solution) x 100
15% = (x grams sodium chloride / 175 g total) x 100
Solving for x will give you the amount of NaCl:
x = 15 x 175 / 100
x = 26.25 grams NaCl
So, now you know how much salt is needed. The solution consists of the sum of the amount of salt and water. Simply subtract the mass of salt from the solution to obtain the mass of water that is required:
mass of water = total mass - mass of salt
mass of water = 175 g - 26.25 g
mass of water = 147.75 g
Mole Concept
Mole Concept is equal to 6.023 x 1023 entities present in a substance. It is also called Avagadro’s number. Mole = given mass of substance to its atomic or molecular mass.
Example 1: Calculate the number of moles for the following:
(i) 52 g of He (finding mole from mass)
(ii) 12.044 × 1023 number of He atoms (finding mole from number of particles).
Solution:
No. of moles = n
Given mass = m
Molar mass = M
Given number of Particles = N
Avogadro number of Paticles = N0
(i) Atomic mass of He = 4u
Molar mass of He = 4g
Thus, the number of moles = given mass/molar mass
n = m/M = 52/4 = 13
(ii) we know,
1 mole = 6.022 × 1023
The number of moles
= given number of particles/Avogadro number
n=N/N0= 12.044 × 1023/6.022 × 1023= 2
Example 2: Calculate the mass of the following:
(i) 0.5 mole of N of molecule)
(ii) 0.5 mole of N atoms (mass from mole of atom)
(iii) 3.011 × 1023 number of N atoms (mass from number)
(iv) 6.022 × 10 23 number of N2 molecules (mass from number)
Solution:
(i) mass = molar mass × number of moles
m = M x n = 28 0.5 = 14 g
(ii) mass = molar mass × number of moles
⇒ m = M × n = 14 × 0.5 = 7 g
(iii) The number of moles, n
= given number of particles/Avogadro number =N/N0
= 3.011 × 1023/6.022 × 1023
m=M x n =14 x 3.011 × 1023/6.022 × 1023
=14×0.5 =7 g
(iv) n =N/N0
m= M x N/N0= 28 x 6.022 × 1023/6.022 × 1023
=28×1=28 g
5. Molecular Mass And Mole Concept
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
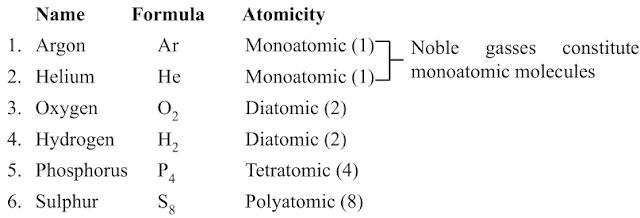
Atomicity
→ The number of atoms present in one molecule of an element is called its atomicity.
CHEMICAL FORMULAE
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Chemical Formulae
→ It is the symbolic representation of the composition of a compound.
• Characteristics of chemical formulae
→ The valencies or charges on ion must balance.
→ When a compound is formed of metal and non-metal, symbol of metal comes first. E.g., CaO
NaCl, CuO.
→ When polyatomic ions are used, the ions are enclosed in brackets before writing the numbe
show the ratio. E.g., Ca(OH)2, (NH4)2SO4
• Rules for writing chemical formulae
(i) We first write symbols of elements which form compound.
(ii) Below the symbol of each element, we should write their valency.
(iii) Now cross over the valencies of combining atoms.
(iv) With first atom, we write the valency of second atom (as a subscript).
(v) With second atom, we write valency of first atom (subscript).
MOLECULAR MASS
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Molecular Mass
→ It is the sum of atomic masses of all the atoms in a molecule of that substance.
Example: Molecular mass of H2O = 2×Atomic mass of Hydrogen + 1×Atomic mass of Oxygen
So, Molecular mass of H2O = 2×1 + 1×16 = 18 u
Formula Unit Mass
→ It is the sum of atomic mass of ions and atoms present in formula for a compound.
Example: In NaCl,
Na = 23 a.m.u.
Cl = 35.5 a.m.u.
So, Formula unit mass = 1×23
+ 1×35.5 = 58.5 u
IONS AND MOLE CONCEPT
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Ions
→ An ion may be defined as an atom or group of atoms having positive or negative charge.
→ Some positively charged ions : Na+ , K+ , Ca2+ , Al3+
→ Some negatively charged ions : Cl- (chloride ion), S2- (sulphide ion), OH-(hydroxide ion),
SO42- (sulphate ion)
• We can classify ions in two types:
(i) Simple ions
Mg2+ (Magnesium ion)
Na+(Sodium ion)
Cl-(Chloride ion)
Al3+ (Aluminium ion)
(ii) Compound ions
NH4+ (Ammonium ion)
CO32- (Carbonate ion)
SO42- (Sulphate ion)
OH- (Hydroxide ion)
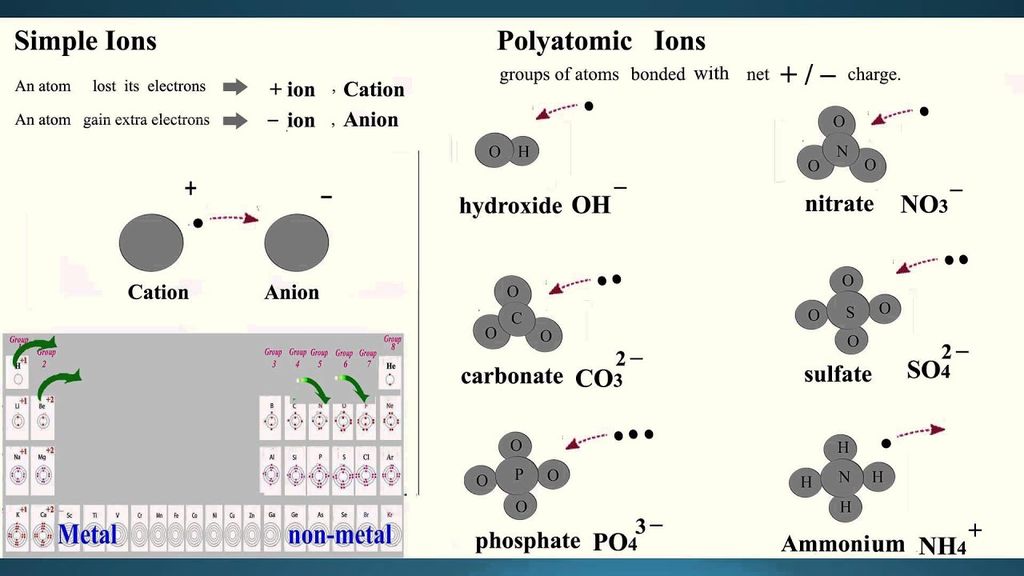
• Chemical Formulae of Ionic Compounds (Polyatomic)
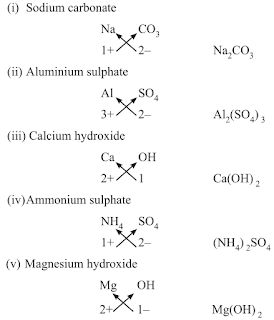
Mole Concept
→ A group of 6.022×1023 particles (atoms, molecules or ions) of a substance is called a mole of
substance.
→ 1 mole of atoms = 6.022×1023 atoms
→ 1 mole of molecules = 6.022 × 1023 molecules
Example, 1 mole of oxygen = 6.022×1023 oxygen atoms
Note: 6.022×1023 is Avogadro Number (L).
→1 mole of atoms of an element has a mass equal to gram atomic mass of
the element.
Molar Mass
→ The molar mass of a substance is the mass of 1 mole of that substance.
→ It is equal to the 6.022×1023 atoms of that element/substance.
Examples:
(a) Atomic mass of hydrogen (H) is 1 u. Its molar mass is 1 g/mol.
(b) Atomic mass of nitrogen is 14 u. So, molar mass of nitrogen (N) is 14 g/mol.
(c) Molar mass of S8 = Mass of S×8 = 32×8 = 256 g/mol
(d) Molar mass of HCl = Mass of H + Mass of Cl = 1 = 35.5 = 36.5 g/mol
Important Formulae -
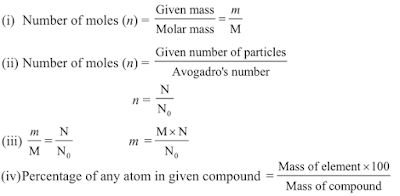

 Science Made Easy
Science Made Easy
 ACERISE INDIA
ACERISE INDIA
