- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
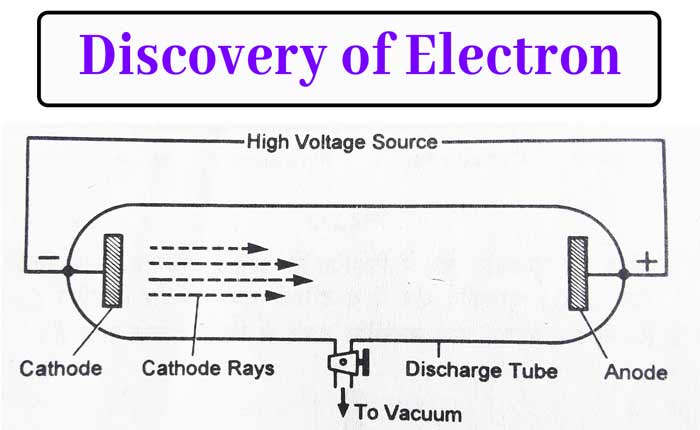
Discovery of Electrons – Cathode Rays (By J. J. Thomson)
→ Thomson explained presence of electrons by cathode rays experiment.
Facts about Electrons
→ Charge on electron = −1.6 × 10-19 C (C = Coloumb)
(As calculated by Robert E. Millikan)
→ Mass of electron = 9.1 × 10-31 kg
Chapter 4
Structure of an Atom
Charged particles in Matter
• Atoms are the basic building blocks of matter.
• Different kinds of matter exist because there are different kinds of atom
present in them.
Postulates of Dalton’s Atomic Theory
• All matter is made up of tiny, indivisible particles called atoms.
• All atoms of a specific element are identical in mass, size, and other properties. However, atoms of different element exhibit different properties and vary in mass and size.
• Atoms can neither be created nor destroyed. Furthermore, atoms cannot be divided into smaller particles.
• Atoms of different elements can combine with each other in fixed whole-number ratios in order to form compounds.
• Atoms can be rearranged, combined, or separated in chemical reactions.
• John Dalton considered atom to be an individual entity, but his concept had to be discarded at the end of 19th century, when scientist through experiments able to find existence of charge (electrons & protons) and neutral particles (neutrons) in the atom. These particles were called sub atomic particles.
• The discovery of electron and proton is credited to J.J. Thomson and E. Goldstein, respectively.
• J.J. Thomson proposed that electrons are embedded in a positive sphere
• Electron was represented as 'e-' and proton as 'p+'. The mass of a proton is taken as one unit and its charge as plus one where the mass of an electron was considered to be negligible and its charge minus one
• It seemed that atom consisted of electrons and protons which balanced their charges mutually.
- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
Chapter-4
Structure of the Atom
Structure of the Atom Introduction
As you all know, we come across different substances around us that constitute matter. This matter is made up of small particles called atoms. Let us know more about atom, its structure and its constituents. Matter: is anything that occupies space and has mass. It consists of tiny particles which were called ‘parmanu’ but later on this name was replaced by ‘atom’.
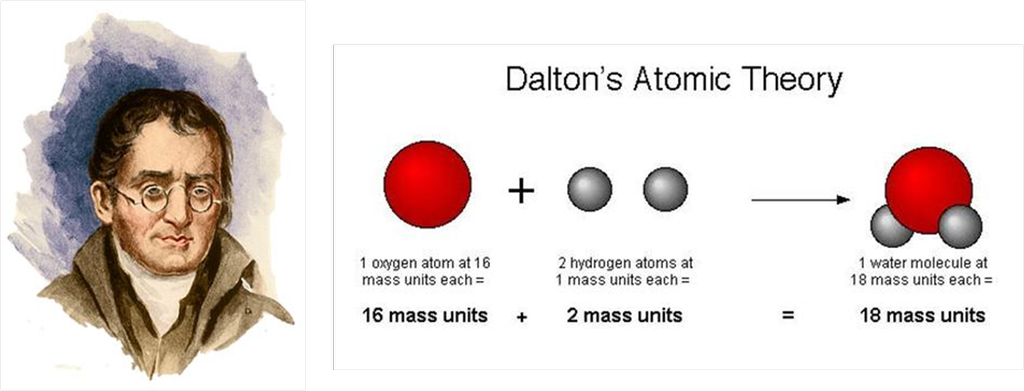
Dalton’s Atomic Theory
The scientist, Dalton was the first one to actually formulate all information of an atom in a theoretical form as Dalton’s Atomic Theory. He was born in 1766 and died in 1844. His theory was published in 1808. His full name was John Dalton
According to this theory, the following observations were made
1. All matter is made up of small particles called atoms.
2. Atom is invisible.
3. Atom is indivisible.
4. Atoms of an element are alike in all aspects, that is, if we talk about sodium then all the atoms of sodium will be the same in all aspects.
5. Atoms of different elements are different, that is, if we talk about sodium and potassium, then, the atoms of both are going to be different but same among themselves.
6. Atoms of different elements combine in a fixed simple whole number ratio to form compounds.
7. Atoms can neither be created nor destroyed, that is, the origin of atoms is not known.
Drawbacks of Dalton’s Atomic Theory
There were certain limitations as observed by other scientists. They observed the following
1. According to Dalton, an atom was indivisible but later on, it was proved that atom can be subdivided into sub atomic particles called electrons, protons & neutrons.
2. Atoms of the same element can somehow differ from each other. This was proved due to the existence of isotopes in nature.
3. Similarly, atoms of different elements can be the same. This came into notice due to the existence of isobars in nature.
4. According to it, whenever the compound is formed, it is formed as a result of the combination of atoms in a fixed simple ratio. But it has been seen that the ratio might not always be simple. For example, in sucrose that is C12H22O11, the ratio is not a simple ratio.
So, these drawbacks led to failure of Dalton’s theory of an atom.

 Science Made Easy
Science Made Easy
 ACERISE INDIA
ACERISE INDIA
