- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Rutherford’s Atomic Model
→ In his famous ‘α-ray Scattering Experiment’, Rutherford bombarded α-ray (Helium nucleus 2 H
upon thin gold foil.
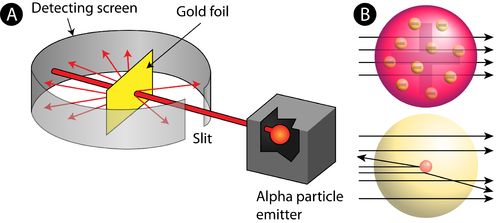
• Observations made by Rutherford in his experiment:
(i) Most of α-particles passed through gold foil undeflected.
(ii) Some of the α-particles deflected by foil by small angles.
(iii) One out of every 12000 particles appeared to rebound.
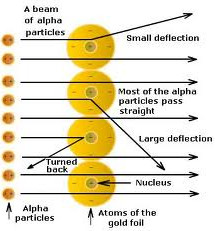
• Conclusions made by Rutherford:
(i) Atom consists of predominantly empty space as most of α-particles passed through gold foil undeflected.
(ii) Atom contains centrally placed positively charged nucleus (carrying positively charged particles), because few α-particles suffered deflected and very few i.e., one in 12000 bounced back.
(iii) Since a minute fraction of α-particles suffered deflections and very few bounced back, this
to conclusion that most of the space an atom is empty and the space occupied by nucleus is negligible compared to this empty space.
→ Size of nucleus was about 10-5 times that of size of atom.
(iv) Whole of the atomic mass concentrated in the nucleus.
• Features of Rutherford proposed model of atom:
(i) There is positively placed nucleus in an atom. Nearly all the mass resides in nucleus (Proton + Neutron).
(ii) Electrons revolves round the nucleus in well defined orbits.
(iii) Size of nucleus is very small compared to the size of atom.
• Drawbacks of Rutherford’s Model (Unstability of Atom)
→ According to Rutherford, electrons revolve round the nucleus in well-defined orbits, but elec
being charged particles will lose their energy and finally will fall into the nucleus.
→ This will make atom highly unstable.
→ This was the major drawback of Rutherford which was unexplained by him.
→ To overcome drawbacks of Rutherford’s Model, Neil Bohr in 1912 proposed modified model of
structure of atom.
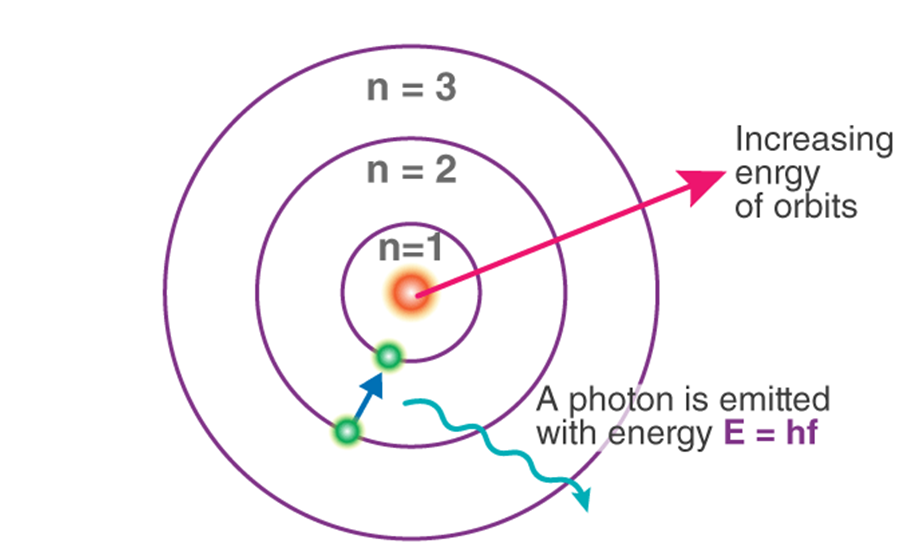
Assumption made by Neil Bohr
→ Only certain special orbits known as discrete orbits of electrons are allowed inside the atom
→ While revolving in discrete orbits, the electrons do not radiate energy.
→ Energy is emitted or absorbed by an atom only when an electron moves from one orbit to another.

 Science Made Easy
Science Made Easy
