- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Colloidal solution and its properties
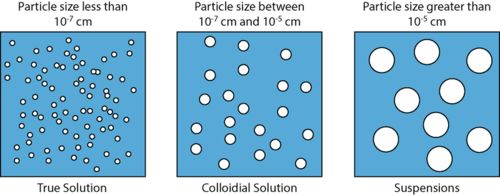
Colloid solution is heterogeneous mixture in which the size of particles lies between the true solutions and suspensions.
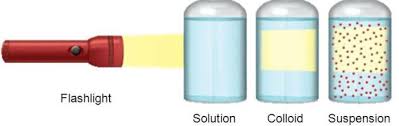
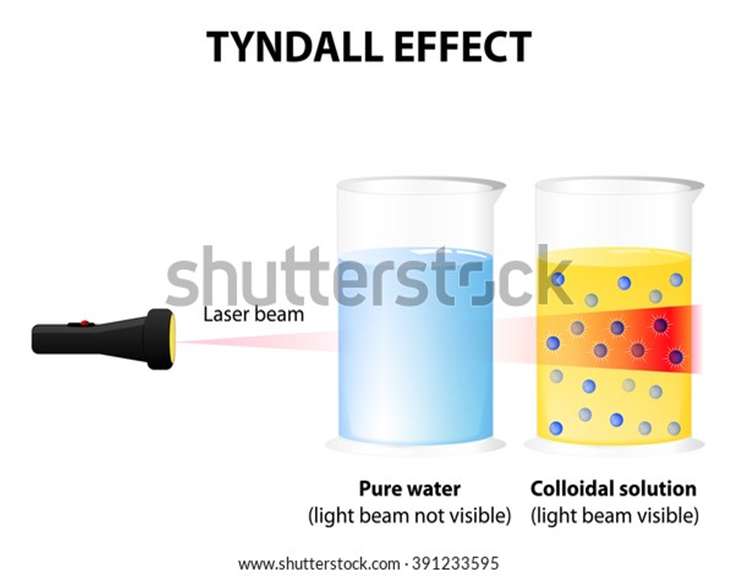
• Colloidal particles can easily scatter a beam of visible light. This phenomenon is called
Tyndall effect.
Properties of colloidal solution:
1. The particles of colloid can’t be seen by naked eyes individually.
2. It is a heterogeneous mixture and thus solute and solvent can’t be separated by filter paper.
3. Size of particles is smaller than suspensions but greater than solutions (1 nm to 100 nm).
4. It is a stable mixture. Particles do not settle down at the bottom over a period of time.
5. They do not settle down when left undisturbed which means colloid is quite stable.
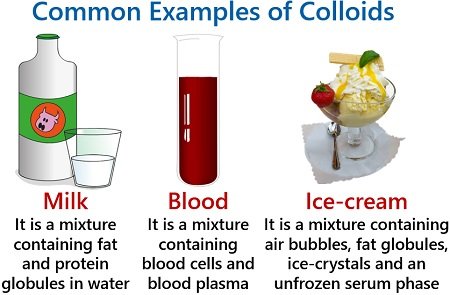
Some common examples of colloids (in the table)
What is a suspension?
In which solids are dispersed in liquids, are called suspensions.
A suspension is a heterogeneous mixture
Particles of a suspension are visible to the naked eye.
Properties of a Suspension
• Suspension is a heterogeneous mixture.
• The particles of a suspension can be seen by the naked eye.
- The particles of a suspension scatter a beam of light passing through it and make its path visible.
• The solute particles settle down when a suspension is left undisturbed, that is, a suspension is unstable.
WHAT IS A COLLOIDAL SOLUTION?
A colloidal solution is a heterogeneous mixture, for example, milk.
Because of the small size of colloidal particles, we cannot see them with naked eyes.
These particles can easily scatter a beam of visible light.
Tyndall effect
The scattering of a beam of light is called the Tyndall effect
The Tyndall effect can also be observed when a fine beam of light enters a room through a small hole.
This happens due to the scattering of light by the particles of dust and smoke in the air.
Observation of Tyndall effect
The Tyndall effect can be observed when sunlight passes through the canopy of a dense forest.
Properties of a colloid.
A colloid is a heterogeneous mixture.
The size of particles of a colloid is too small to be individually seen by naked eyes.
Colloids are large enough to scatter a beam of light passing through it and make its path visible.
They do not settle down when left undisturbed, that is, a colloid is quite stable.
They cannot be separated from the mixture by the process of filtration.
Dispersing medium
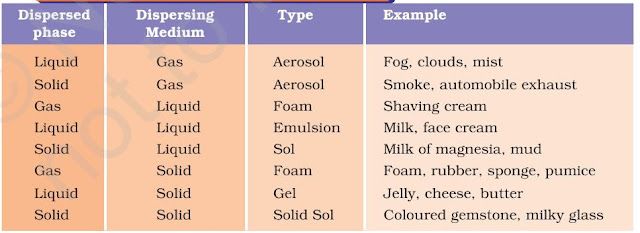
The components of a colloidal solution are the dispersed phase and the dispersion medium.
The solute – like component or the dispersed particles in a colloid form the dispersed phase, and the component in which the dispersed phase is suspended is known as the dispersing medium.
- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
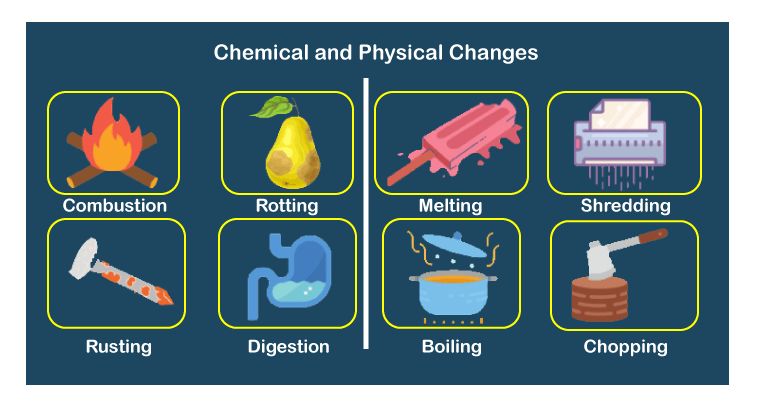
Physical and chemical Change
We observe different kind of changes around us like if you forget to keep the milk inside the fridge in summers it gets rancid, likewise when you keep ice out of the refrigerator, you observe the water around it and finally the ice gets converted into water. These all are changes that are taking place around us. But some changes can be reversed like water can be converted back to ice but rancid food item can not be made fresh again. Let us learn in detail about these changes.
1. Physical change
2. Chemical change


 Science Made Easy
Science Made Easy
 ACERISE INDIA
ACERISE INDIA
