- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Effect of change of pressure
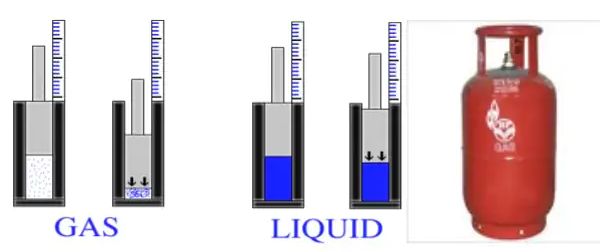
→ Gases can be liquefied by applying pressure and reducing the temperature. When a high pressure is applied to a gas, it gets compressed and if the temperature is lowered, the gas is liquefied.
→ Solid CO2 gets converted directly to gaseous state on decrease of pressure to 1 atmosphere without coming into liquid state. This is the reason that solid carbon dioxide is also known as dry ice

 Science Made Easy
Science Made Easy
