- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Solution and its properties
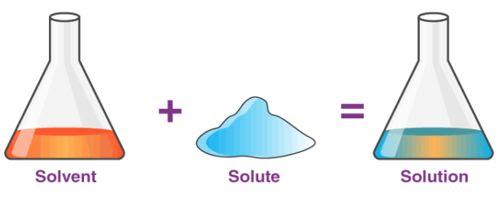
A solution is a homogeneous mixture of two or more substances. Ex: Lemonade, soda water etc
A solution has two components:
(i) Solvent
(ii) Solute
(i) Solvent: The component of the solution that dissolves the other component in it (usually the component present in larger amount) is called the solvent.
(ii) Solute: The component of the solution that is dissolved in the solvent (usually present in less quantity) is called the solute.
Properties of Solution:
1. A solution is a homogeneous mixture.
2. The particles of a solution are smaller than 1 nm
(10-9 ) in diameter which cannot be seen by naked
eyes.
3. They do not scatter a beam of light passing through the solution that is they don’t show tyndall effect. So, the path of light is not visible in a solution.
4. The solute particles cannot be separated from the mixture by the process of filtration.
5. The solution is stable and solute particles do not settle down when left undisturbed.
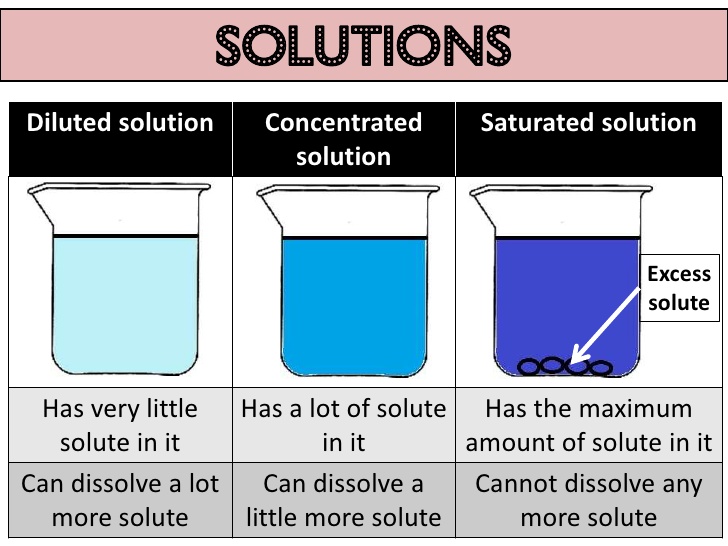
Concentration of a solution
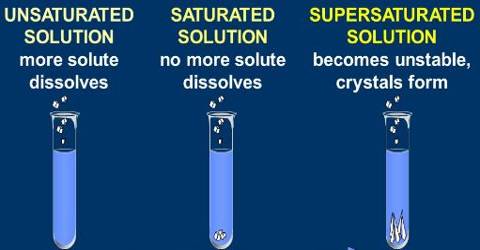
(i) Saturated solution: When no more amount of solute can be dissolved in a solution at a give temperature, it is called a saturated solution.
(ii) Unsaturated solution: When more amount of solute can be dissolved in a solution at a give temperature, it is called a saturated solution.
(ii) Solubility: The amount of the solute present in the saturated solution at the given temperature
called its solubility.
The concentration of a solution is the amount of solute present in a given amount (mass or volume) of solution. Also, the amount of solute dissolved in a given mass or volume of solvent is called concentration of solution.
Concentration of solution = Amount of solute/Amount of solvent or Amount of solute/Amount in
solution (Here, amount means mass or volume).
Two methods of finding concentration of solution:
(i) Mass by mass percentage of a solution = (Mass of solute/Mass of solution) ×100
(ii) Mass by volume percentage of a solution = (Mass of solute/Volume of solution) ×100
But when we see around us, we observe most of the matter around us exists as mixtures of two or more pure components. For example: Sea water, Air etc.
WHAT IS A MIXTURE?
It is a form of matter in which two or more elements or compounds combine physically in any proportion by weight.
Characteristics of Mixture
- Mixture may be homogeneous and heterogeneous.
- Mixture does not have a fixed melting point.
- In a mixture, the different constituents combine physically in any proportion by mass.
- The constituents of a mixture do not lose their identical property.
- Usually, no energy change take place during the formation of a mixture.
Types of Mixtures
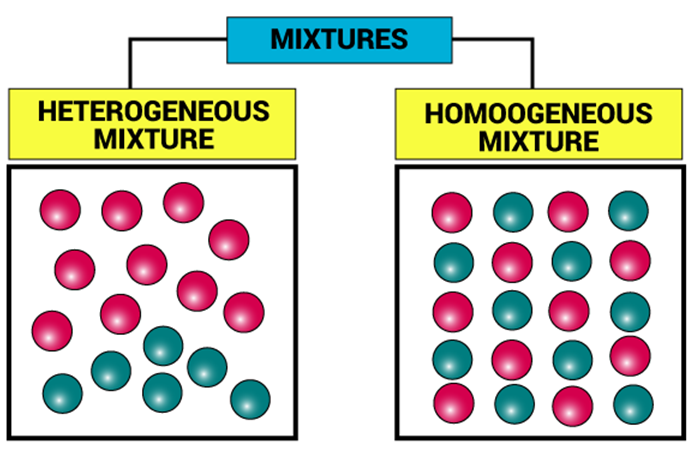
- Homogeneous mixture: A mixture which has same composition throughout. It has no visible boundaries of separation between the various constituents Solutions are homogeneous mixtures. For example, Detergent in water, Sugar in water, Ice cream etc.
- Heterogeneous mixture: A mixture which has different compositions in different parts. These types of mixtures have visible boundaries of separation between the various constituents. For example, Oil in water, Fruit salad, Sand in water etc.
- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
Impure substance
The substances that are formed by different kinds of particles are called impure substances. For example : Mixtures are impure substances.
Mixtures
You must have eaten pakoras at home. Have you noticed how your mother prepares it. She makes a batter containing besan, water, spices, etc. These constituents can be added in any ratio. In all ratios, they will form pakoras but the difference will be that sometimes, they will be hard to touch or the batter may be too loose to fry.
Mixtures: They are formed when two or more substances are simply mixed in any ratio and are not chemically combined with each other.

Characteristics of a mixture are as follows
- They can be homogeneous or heterogeneous in nature, that is, the constituents can be seen to have visible boundaries or they may appear to mix thoroughly.
- The properties of a mixture are the same as that of its constituents.
- The constituents can be separated by physical methods.
- Their formation does not require or release energy as there is no bond formation or breakage involved.
- The properties of mixtures like melting point & boiling point are not fixed.
Types of Mixtures
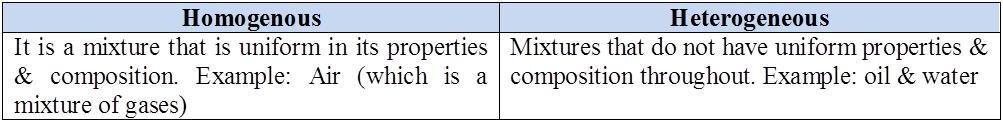
Solutions
We all like drinking sweet lemon water in summers as it gives a cooling effect. It is made of lemon, water, ice, salt and sugar. These all components when mixed, form sweet lemon water mixture which is also called a solution. These components can be separated by using different techniques. Let us learn what exactly solutions are and their types.
A Solution is a homogenous mixture of two or more substances. To form it, we can add 2 or more components. These components can be in any ratio but are simply mixed, that is not chemically combined. Generally, two components of solutions are seen and are called ‘Solute’ and ‘Solvent’.
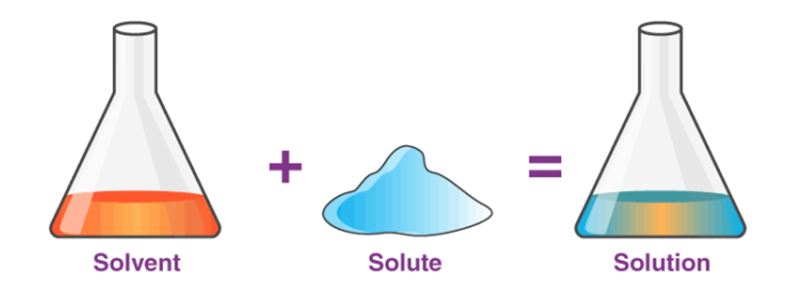
Solute
It is the constituent which is present in a comparitively lesser amount and gets dissolved in the solvent.
Solvent
It is the constituent present in more amount and it has the ability to dissolve the solute in it. If you dissolve salt in water, then the salt is in lesser quantity and it gets dissolved. So, here solute is salt and solvent is water
Characteristics of solutions are as follows
- They are homogeneous.
- Their Composition can vary.
- The size of particles is very small.
- They do not scatter light.
- They can be separated by physical methods.
Types of solutions
1. On the basis of dissolving nature of liquids
You must have noticed that when you dissolve sugar or salt in water, it just vanishes after a few seconds or a minute. The reason is that it gets mixed in water. But if we add oil in water, it does not vanish and is seen floating on its surface. It is due to this reason that some substances can mix into each other and some do not. Let us learn about it in detail
Miscible, immiscible & partially miscible solutions
Miscible liquids
The liquids that completely mix into each other to form a solution are miscible liquids. For example: alcohol when added to water gets completely mixed.

Partially miscible liquids
The liquids which can dissolve in another liquid only up to some extent to form a solution are partially miscible liquids. For example: ethylene glycol in chloroform.
Immiscible liquids
The liquids which do not mix into each other are immiscible liquids. For example: oil & water.

2. On the basis of nature of solvent
We have seen that it’s not only water in which we can dissolve substances, we can make use of other substances as well. For example- carbon tetrachloride, Benzene, alcohol, etc. and many more reagents. So, we have another classification based on the nature of solvent.
Aqueous and Non aqueous solutions
Aqueous
The solution in which the solvent is water is an aqueous solution. Example: salt solution
Non-aqueous solutions
The solution in which the solvent is other than water is a non-aqueous solution. Example: alcohol, benzene etc.
3. Classification on the basis of solubility power of solvent
You must have seen that if you take one glass of water at room temperature and you add 1 spoon of sugar to it, it dissolves. But if you keep on adding sugar to the same solution, a point will be reached when it stops dissolving sugar in it and the sugar starts getting deposited at the bottom. The reason being that each solvent has some solubility power and it can dissolve only up to that limit. Let us study about it.
Saturated, unsaturated and supersaturated solutions
Saturated Solution
The solution that dissolves as much solute as it is capable of dissolving is a saturated solution.
Unsaturated solution
The solution in which more quantity of solute can be dissolved without increasing its temperature is an unsaturated solution.
Supersaturated
The solution in which the solvent dissolves an amount of solute greater than its solubility. It is formed at high temperature and then slowly cooling it to lower its solubility.
Solubility
Is the amount of solute that can be dissolved in a given amount of solvent at a particular temperature.
Factors affecting solubility
- Nature of solute.
- Nature of solvent.
- Temperature.
Please Note:
- On lowering temperature, solubility of liquids & solid decreases & solubility of gas remains unaffected.
- On increasing pressure, solubility of gas increases & for solid and liquid, it remains unaffected.
4. Classification on the basis of size of solute particles
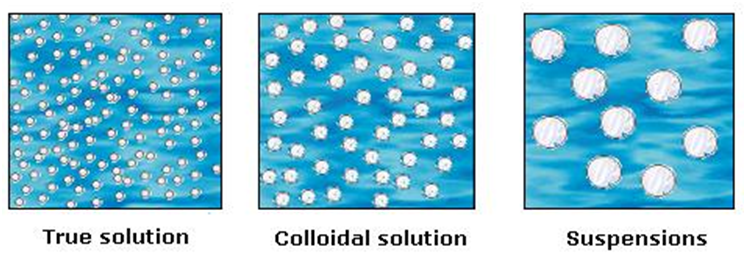
You must have made a solution of sand in water, sugar in water and milk. They don’t look the same. Let us predict the nature of these solutions
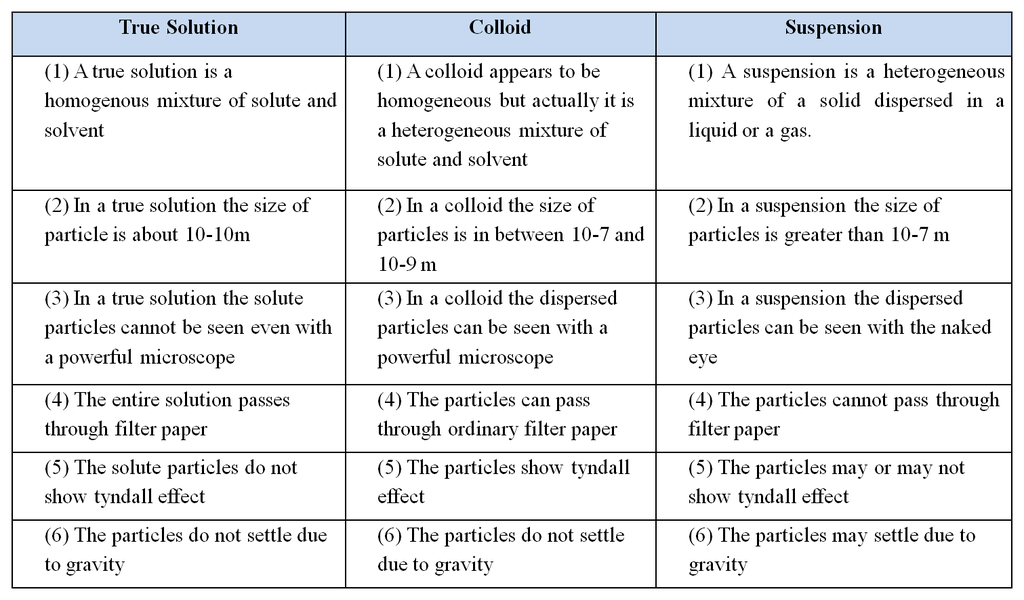
Difference between a true solution, colloid and a suspension

 Science Made Easy
Science Made Easy
 ACERISE INDIA
ACERISE INDIA
