- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Chemical Formulae
→ It is the symbolic representation of the composition of a compound.
• Characteristics of chemical formulae
→ The valencies or charges on ion must balance.
→ When a compound is formed of metal and non-metal, symbol of metal comes first. E.g., CaO
NaCl, CuO.
→ When polyatomic ions are used, the ions are enclosed in brackets before writing the numbe
show the ratio. E.g., Ca(OH)2, (NH4)2SO4
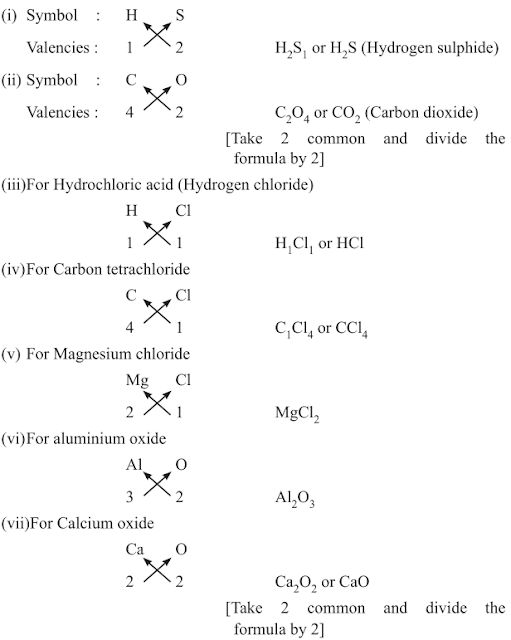
• Rules for writing chemical formulae
(i) We first write symbols of elements which form compound.
(ii) Below the symbol of each element, we should write their valency.
(iii) Now cross over the valencies of combining atoms.
(iv) With first atom, we write the valency of second atom (as a subscript).
(v) With second atom, we write valency of first atom (subscript).

 Science Made Easy
Science Made Easy
