Chemical Formulae
Rules:
(i) The valencies or charges on the ion must balance.
(ii) Metal and non-metal compound should show the name or symbol of the
metal first.
e.g., Na+ Cl– → NaCl
(ii) If a compound consists of polyatomic ions. The ion is enclosed in a bracket before writing the number to indicate the ratio.
e.g., [SO4]2- → polyatomic radical
H1+ SO42- → H2SO4
Molecular Mass
It is the sum of the atomic masses of all the atoms in a molecule of the substance. It is expressed in atomic mass unit (u).
Formula Unit Mass
It is the sum of the atomic masses of all atoms in a formula unit of a compound. The constituent particles are ions.
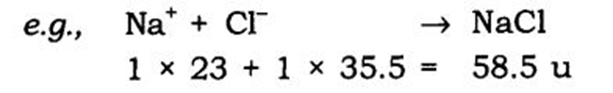
Mole Concept
Definition of mole: It is defined as one mole of any species (atoms, molecules, ions or particles) is that quantity in number having a mass equal to its atomic or molecular mass in grams.
1 mole = 6.022 x 1023 in number
Molar mass = mass of 1 mole → is always expressed in grams and is also known as gram atomic mass.
l u of hydrogen has → 1 atom of hydrogen and 1g of hydrogen has → 1 mole of hydrogen
= 6.022 x 1023 atoms of hydrogen.

 Science Made Easy
Science Made Easy
 ACERISE INDIA
ACERISE INDIA
