Chapter 3
Atoms & Molecules
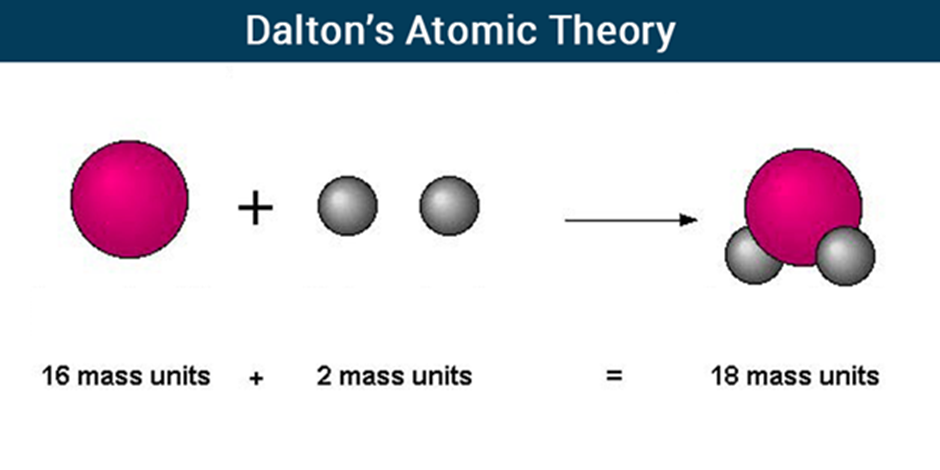
Dalton’s Atomic Theory
The matter is made up of indivisible particles known as atoms.
The properties of all the atoms of a given element are the same, including mass. This can also be stated as all the atoms of an element have identical mass and chemical properties; atoms of different elements have different masses and chemical properties.
Atoms of different elements combine in fixed ratios to form compounds.
Atoms are neither created nor destroyed. The formation of new products (compounds) results from the rearrangement of existing atoms (reactants) in a chemical reaction.
The relative number and kinds of atoms are constant in a given compound.
Laws of Chemical Combination
Given by Lavoisier and Joseph L. Proust as follows:
Law of conservation of mass
According to the law of conservation of mass, matter can neither be created nor destroyed in a chemical reaction. It remains conserved.
Mass of reactants will be equal to the mass of products.
Law of constant proportions
A pure chemical compound contains the same elements combined together in a fixed proportion by mass is given by the law of definite proportions.
For e.g., If we take water from a river or from an ocean, both have oxygen and hydrogen in the same proportion.
Atom
Atoms are the smallest particles of an element which can take part in a chemical reaction.
Size of an atom: atomic radius is measured in nanometres.
The atomic symbol has three parts: -
The symbol X: the usual element symbol
The atomic number A: equal to the number of protons
The mass number Z: equal to the total number of protons and neutrons in an element.

 Science Made Easy
Science Made Easy
 ACERISE INDIA
ACERISE INDIA
