PHYSICAL NATURE OF MATTER
- Books Name
- EduMple Institute of Learning Science Book
- Publication
- EduMple Learning
- Course
- CBSE Class 9
- Subject
- Science

PHYSICAL NATURE OF MATTER
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Physical Nature of Matter
→ Matter is made up of particles. All matter constitute of very small particles. These small particles are called atoms.
→ These particles of matter are too small so they cannot be seen by naked eyes or simple microscope.
→ Particles of matter are continuously moving as they posses kinetic energy, with the increase in temperature the kinetic energy of particles also increases so particle moves faster.
characteristics - shape, volume, density
- Books Name
- EduMple Institute of Learning Science Book
- Publication
- EduMple Learning
- Course
- CBSE Class 9
- Subject
- Science
Learning Objective
To describe the solid, liquid and gas phases.
Water can take many forms. At low temperatures (below 0oC), it is a solid. When at "normal" temperatures (between 0oC and 100oC), it is a liquid. While at temperatures above 100oC, water is a gas (steam). The state that water is in depends upon the temperature. Each state has its own unique set of physical properties. Matter typically exists in one of three states: solid, liquid, or gas.
1. Matter and its Classification
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Diffusion
→ Brownian Motion: The zig-zag or random path travelled by the particles of matter is called Brownian motion.
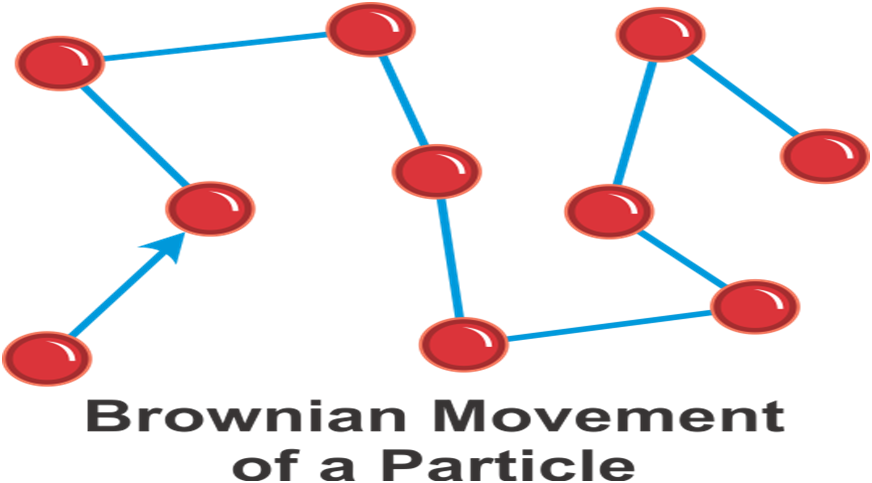
→ Intermixing of particles of two different types of matter on their own is called diffusion.
The rate of diffusion increases on increasing the temperature of the diffusing substance (by heating).
Example - The colour of ink spreading in water due to diffusion of particles of water.

1. Matter and its Classification
- Books Name
- Mayank classes Science Book
- Publication
- Mayank classes
- Course
- CBSE Class 9
- Subject
- Science
rew
1. Matter and its Classification
Chapter 1
Matter in our surroundings
Introduction
- Everything in this universe is made of materials which scientist has names ‘matter’.
- The matter is made up of very small tiny particles. It is not continuous but is particulate.
- The matter is anything that occupies space and has mass.
- Particles of matter have space between them and are continuously moving.
- Particles of matter attract each other.
PHYSICAL NATURE OF MATTER
- Matter is made up of particles
If we take a 100 mL beaker, filling half of it with water and dissolve some salt/sugar. We will observe that there is no rise in water level and the salt/sugar has spread throughout the water (shown in the fig).
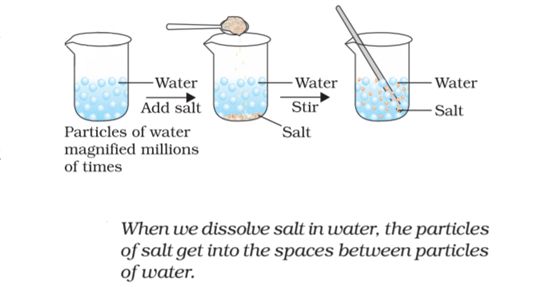
- How small are these particles of matter?
- The particles of matter are very small – they are small beyond our imagination.
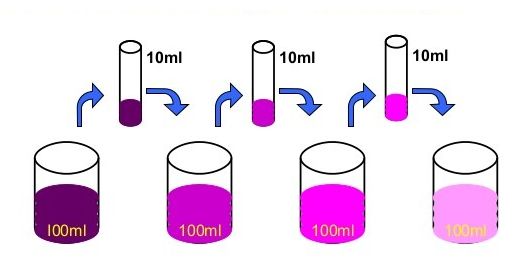
If we dissolve few crystals of potassium permanganate in about 1000mL of water, we will see the colour has changed. It shows that there must be millions of tiny particles in just one crystal of potassium permanganate, which keep on dividing themselves into smaller and smaller particles.
1. Matter and its Classification
- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
Chapter-1
Matter in Our Surroundings

Matter in Our Surroundings
Introduction
Let us know about the meaning of the title of this chapter “matter in our surroundings”. So to know about it we need to understand the term ‘surrounding’ first. Do you know what is meant by the word ‘surrounding’? Surrounding can be anything that is around us like air, clouds, buildings, water bodies, etc. These all are surroundings. Do you know that all the things that surround us are matter? Anything around us that has some mass and occupies certain space when we keep it, is called matter. In this chapter, we will be studying matter, its properties and much more.
Matter and its classification
Matter can be anything that occupies Space and has some Mass, (mass may be small or more doesn’t matter). Like we have LPG cylinder. Although it is filled with air only, but still it is too heavy. So, that is also matter as it has mass and occupies space. Even air is matter. If you see around, you will find that everything surrounding you is matter.
Classification of matter
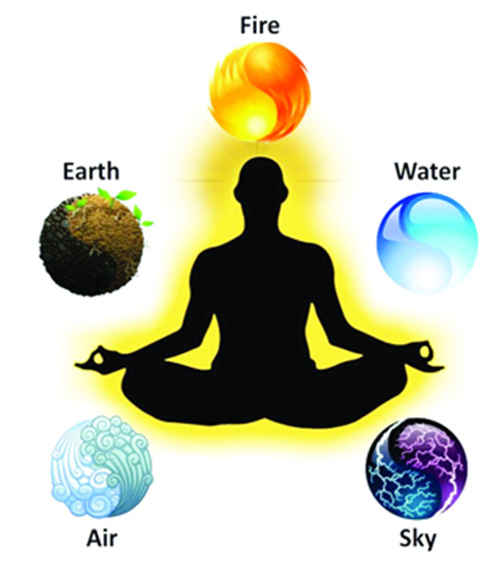
Earlier when Matter was classified, then the ancient scientists said that matter was made up of five basic Elements called: Air, Fire, Earth, and Water & Sky. These five components are called as “panchtatva”.
But with the advancement in the field of science, it was concluded that matter can be classified on the basis of two main properties that is physical and chemical and therefore there are two main categories of matter, as follows –
Classification of Matter
- Chemical Classification
- Physical Classification
Physical and Chemical classification
Physical Classification
It is done on the basis of Physical Properties, i.e. the properties that we can see like rigidity, colour etc.
Chemical Classification
Is done on the basis of Chemical Composition of Matter that means what kind of particles are present, how they react with other, if they do not react then why so. Let’s do some activities to study about Matter. These activities are conducted in order to know more about matter and learn about their properties.
Activity: Do you know how we come to know that matter is made up of small particles? Let’s perform an activity: for this we need the following things
- beaker, common salt, spoon glass rod
1. Fill beaker 3/4th with water mark the level of water on beaker, then add common salt to water.
2. On adding, we see that when we add salt to water it settles down.
3. On stirring, it starts disappearing and then a stage comes when it disappears completely.
Let s see what does this observation proves
- As we are not able to see salt particles, this shows that it is not one continuous state of matter, instead it is formed of small particles.
- Actually, on stirring, the salt particles get dissolved in water but still the level of water remains same. This shows that there are spaces between the particles of water which are occupied by the small salt particles.
Another activity: If we consider about the motion of particles. Do you actually think that they can show movement? If yes, lets track their movement?
In order to perform the activity, the following things are required – Stand, Agarbatti Match box

1. Now let’s take one stick out of packet, hold it on stand, place it in a corner of the room and light it. We will see that its fragrance gets spread evenly in the room.
2. The fragrance can be felt in whole room. It is not confined to one corner. So the observation is that “The whole room starts smelling very good“
The Conclusion to an activity
- Is that particles of matter are not stationary. They are moving continuously and when they drift through air, we can smell the pleasant fragrance.
- The reason behind it is that when we light the stick, the heat energy supplied is taken by the particles, which increases their kinetic energy and this makes the fragrant particles to move rapidly. They easily drift through the air and spread fragrance.
You will be surprised to know the actual phenomenon involved behind this activity- The Phenomenon is called Diffusion.
Diffusion
It is the movement of any substance from a place of higher concentration to a place of lower concentration, or It can also be defined as the intermixing of different substances.
Activity to show diffusion: let’s take two flasks connected to each other by a knob.
Now let’s say on one side there is gas A and on the other side, there is gas B. When knob is closed, both the gases are in their own flasks. But when the knob is opened the two gases intermix rapidl y. As a result, both the flasks will have A and B gas particles. This intermixing is called diffusion. It is best shown by gases as the gas particles have maximum kinetic energy.
Activity to prove: particles of matter attract each other
Let’s take different substances like an iron nail, piece of wood, rubber band

1. Now try to break them by using hammer.
2. As we know, all three belong to same states – solids.
3. Can you guess what we noticed?
4. We noticed that it is easier to hammer down rubber band as compared to wood and iron nail.
5. The reason is that the particles in iron nail are so closely packed that it is difficult to separate those using hammer in comparison to wood or rubber band.
Conclusion of this activity:
In iron, the force of attraction between particles is maximum as particles are closer whereas in others they are not so close.
All these activities lead to the formulation of more information about matter and its properties which lead to the introduction of the Theory of Molecular Structure of Matter.
2. Molecular Theory of Matter
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Characteristics of Particles of Matter
• Particles of matter have space between them
→ Gas can be compressed a lot because of the space between their particles.
Activity:- take some water in a beaker and note its level. Dissolve some salt or sugar in it with the help of a glass rod. The salt dissolves in the water but the level of water dose not change. This is because the particles of salt get the space between the particles of water.
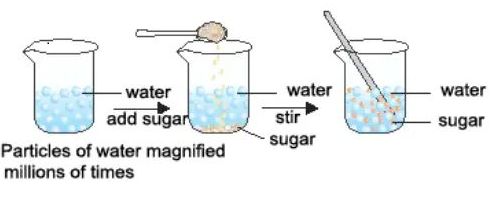
→ Additionally you would notice that there is no rise of water level takes place when one or two teaspoon of sugar/salt is added into a glass of water, this is because sugar/salt particles get adjusted in the space between the particles of water and no rise in the water level comes in result.
• Particles of matter attract each other because of force of attraction.
Activity:- Take an iron nail, a piece of chalk and rubber band. Try breaking them hummering, cutting or stretching. It is more easier to break the chalk, less easier to break the rubber band and difficult to break the iron nail. this is because the particles in the iron nail are held together with greater force than in the rubber band or challk.
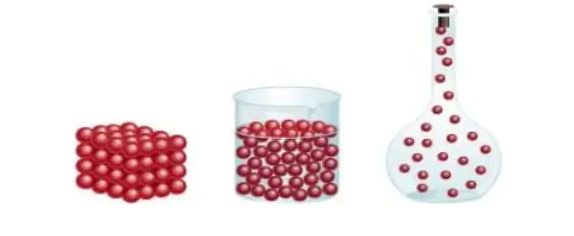
→ Force of attraction between particles of matter keeps the particles bonded together. Therefore attraction between particles of solid is greatest, between particles of liquid is moderate and between particles of gas is lowest.
→ Because of the lowest force of attraction between the particles of gas we can move our hand through
air easily. To move our hand in liquid, such as water, we have to apply some force, but a solid such as wood, we cannot move our hand.
→This is because the force of attraction between particles of gas is almost negligible, in liquid the forces of attraction is moderate but it is greatest in solid.
→ The force of attraction between particles of solid, liquid and gas can be arranged in decreases in order as follows:
Solid > Liquid > Gas
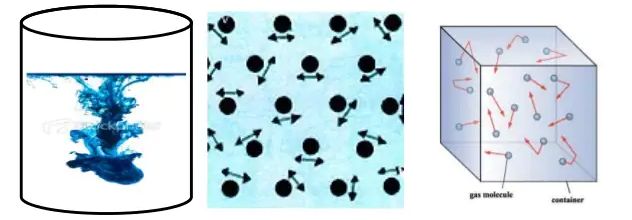
• Particles of matter are continuously moving –
Activity :- Take some water in a beaker and put a drop of blue or red ink slowly along the sides of the beaker. Leave it undisturbed for a few hours. The ink spreads evenly throughout the water due to the movement of the particles of water and ink.
The intermixing of two or more different types of matter on their own is called diffusion.
• Matter is made up of small particles –
Activity :- Dissolve 2 —3 crystals of potassium permanganate in I OOml of water in a beaker. Take lOmI of this solution and dissolve in lOOmI of water. Take lOmI of this solution and dissolve In lOOmI of water. Repeat this process 5— 6 times. This shows that a few crystals of potassium permanganate can colour a large volume of water because there are millions of tiny particles in each crystal
Classification of states of matter
States of Matter
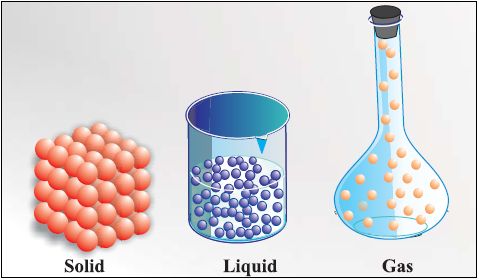
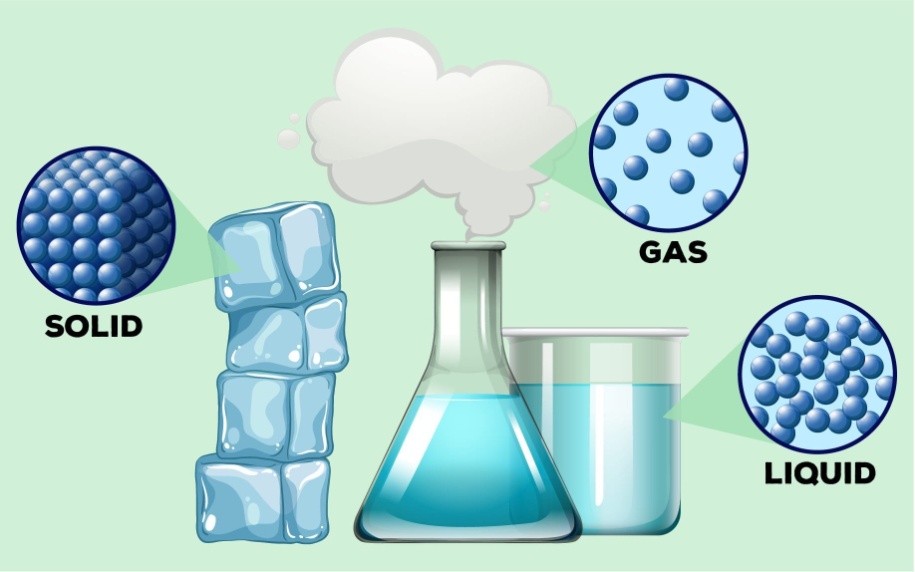
• Solid State
→ The space between the particles is very less.
→ The force of attraction between the particles is strong. Thus, particles in a solid are closely packed.
→ Solids maintain their shape even when they are subjected to external force i.e. they are rigid
→ Solids cannot be compressed.
→ The kinetic energy of the particles is very less and so solids have an orderly arrangement of particles. Therefore, solids have a fixed shape and volume.
• Liquid State
→ The space between the particles is slightly more as compared to solids, but still very less as compared to gases. The particles of a liquid can slip and slide over each other.
→ The force of attraction between the particles is strong enough to hold the particles together not strong enough to hold the particles in a fixed position.
→ Liquids do not have a fixed shape but have a fixed volume. Liquids take up the shape of the container in which they are poured.
→ The kinetic energy of the particles is more than that of solids. Thus, liquids have a disorderly arrangement of particles compared solids.
→ Liquids cannot be compressed much. The compressibility of liquids is almost negligible.
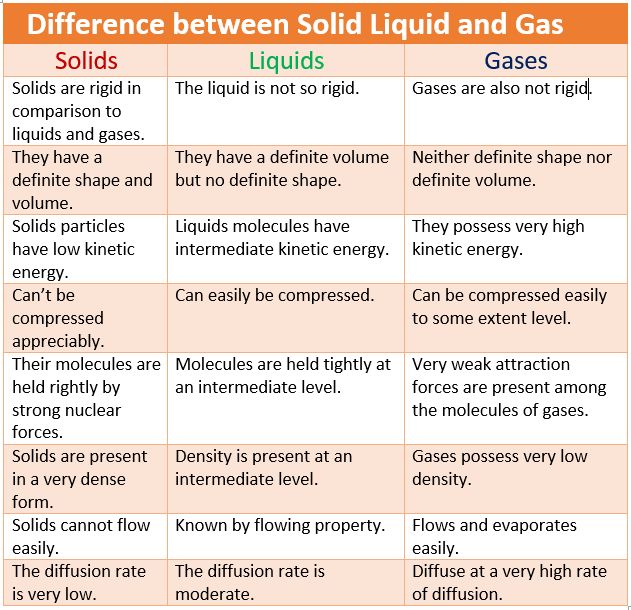
• Gaseous State
→ The particles are much farther apart from one another as compared to solids and liquids.They have a very disorderly arrangement of particles compared to the solids and liquids.
→ The force of attraction between the particles is negligible, hence particles of a gas move free in all the directions.Gases thus can mix or diffuse into other gases.
→ The particles of a gas have maximum kinetic energy. They move with high speed in all directions and can exert pressure on the walls of its container.
→ Gases neither have a definite shape nor a definite volume.They fill up the container completely
→ Gases can be compressed easily. Example: the LPG cylinders used at home and the CNG cylinder used in vehicles.
2. Molecular Theory of Matter
CHARACTERISTICS OF PARTICLES OF MATTER
1. Particles of matter have space between them
2.Particles of matter are co ntinuously moving –
- They possess kinetic energy.
- Increase in temperature also increases the kinetic energy of the particles
- Thus, Particles of matter intermix on their own with each other.
- They do so by getting into the spaces between the particles.
- This intermixing of particles of two different types of matter on their own is called diffusion
- On heating, diffusion becomes faster.
3. Particles of matter attract each other.
- The strength of this force of attraction varies from one kind of matter to another.
2. Molecular Theory of Matter
- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
Molecular Theory of Matter
Theory of molecular structure of matter
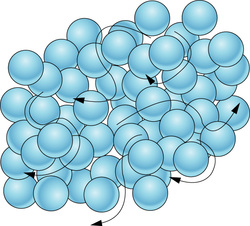
According to this theory,
- Matter is made up of small particles called molecules.
- They are in a state of continuous motion. Due to this, we can say that they possess K.E.
- K.E. Increases with ↑ se in temperature.
- K.E. Is maximum in gases and least in solids.
- The space between molecules is called intermolecular space which is found to be least in solids and maximum in gases.
- There is a force that exists between particles of matter and is called intermolecular force
- Intermolecular force ↓ ses with increase in Intermolecular space
Terminology involved
Before we start with more properties of matter let us know some terms which will be used in this chapter.
- Matter: Anything that occupies space is matter.
- Material: The term is used to describe a particular kind of matter.
Materials are of two Types:
- Homogenous
- Heterogeneous

Homogeneous Materials: Are those that have the same composition and same properties throughout the sample. For example, if you take water in a glass and add salt to it and stir it. It will result in formation of a mixture that has same properties and uniform composition (that is no distinct layers are seen). They mix thoroughly. If you take sample from any part of that mixture, it will show similar properties.
Heterogeneous Materials: are those that have different composition and different properties in different parts of the sample. For example, if we take sand in water, then we will see that inspite of stirring, it does not dissolve in water. It will settle at the bottom of the container and few particles will be seen floating in the water. If you take a sample from any part of it, it will show different properties.
Molecule: Is a term used for particular type of matter that has independent existence in nature like oxygen molecule, carbon dioxide molecule, water molecule, etc.
Molecular structures and properties of solids, liquids and gases
Now let us do Physical Classification of Matter: this classification is done on the basis of physical properties of matter, that is the properties that can be seen or felt by touching, looking etc.
According to it, matter is divided into 3 types:
- Solids
- Liquids
- Gases
Solids
if you take any solid let’s say we consider wood, what properties can you find in it by looking at it.
- First property that we can make out is: That it has fixed shape and volume: we realize this because when wood is kept on the floor or any surface, it occupies definite space.
- Another property that we notice is: It is not compressible: if we it try to squeeze it or change its shape, we cannot do so. This is probably due to the reason that there is no space between solid particles and in order to compress it, there should be space between particles. This is because when we apply force, the particles fill those empty spaces and come closer.
- Another property: They don’t need container to hold them: we don’t have to put an almirah or any other solid in a container. it can be kept as such on the surface.
- They do not flow: This is because when wood is kept at a place, it remains at the same place. It doesn’t flow or move on its own.
- Their diffusion tendency is nil: it is seen that wood doesn’t move from one side of room to another side of room on its own as the particles do not have that much energy that they keep on moving.
Let us try to understand solids on the basis of theory of molecular structure:
Explanation of Solids on the basis of Molecular Structure
If we see any solid we observe that in solids, particles are tightly packed. They are very closely packed due to which there is hardly any space between particles or we can say that intermolecular spaces are very less and when particles are closer, that means the intermolecular forces of attraction are stronger. As a result, solids have fixed shape, volume and can’t be compressed.
Now do you understand that why wood or any other solid doesn’t flow or compress or diffuse and why they possess high density.
Liquids
Let’s sum up the properties of gases in comparison to solids and liquids
- They have neither fixed shape nor fixed volume
- They are highly compressible
- They can flow, diffuse to great extent
- They have very very low density
- They can fill the entire space
Explanation of gases on the basis of molecular structure
In gases the particles are very far apart. Due to this, they have high kinetic energy and keep on moving randomly. As a result, the intermolecular spaces are very large and intermolecular forces are almost nil.
3. Plasma and BEC State, Temperature
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Change of State of Matter

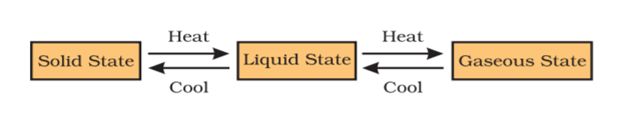
→ The phenomenon of change from one state of matter to another, and then back to the original state is called the interconversion of states of matter.
→ Matter Can Change its State. Water can exist in three states of matter:
• Solid as ice
• Liquid as water
• Gas as water vapour
Effect of Temperature
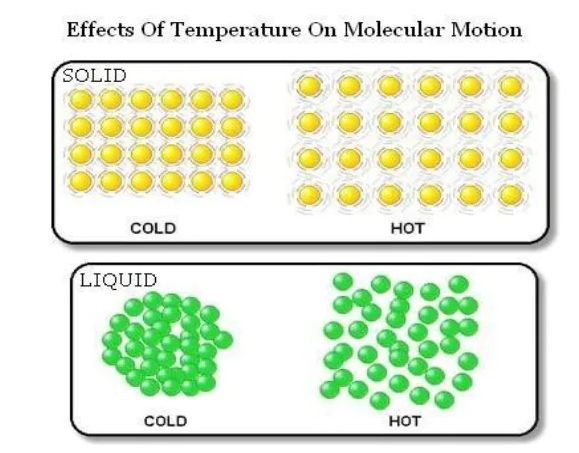
→ On increasing the temperature of solids, the kinetic energy of the particles increases which overcomes the forces of attraction between the particles thereby solid melts and is converted into liquid.
→ The temperature at which a solid melts to become a liquid at the atmospheric pressure is called its
melting point.
The melting point of ice is 273.16 K.
The process of melting, that is, change of solid state into liquid state is also known as fusion.
3. Plasma and BEC State, Temperature
STATES OF MATTER
Matter around us exists in three different states– solid, liquid and gas, dependent on the characteristics of the particles of matter.
1. SOLID STATE – Solids have a definite shape, distinct boundaries and fixed volumes, that is, have negligible compressibility.
- Solids may break under force but it is difficult to change their shape, so they are rigid.
Examples, a pen, a book, a needle and a piece of wooden stick, a granule of sugar.
- There are objects that are solid in state but seems do not follow the above rule but actually they do
- A rubber band changes shape under force and regains the same shape when the force is removed. If excessive force is applied, it breaks.
- A sponge has minute holes, in which air is trapped, when we press it, the air is expelled out and we are able to compress it.
2. LIQUID STATE – liquids have no fixed shape but have a fixed volume.
- They take up the shape of the container in which they are kept.
- Liquids flow and change shape, so they are not rigid but can be called fluid.
- Solids, liquids and gases can diffuse into liquids (e.g. oxygen and carbon dioxide dissolves in water, which helps the survival of aquatic animals and plants)
- The rate of diffusion of liquids is higher than that of solids.
- This is because in the liquid state, particles move freely and have greater space between each other as compared to particles in the solid state.
3. GASEOUS STATE – gases are highly compressible as compared to solids and liquids
- Due to its high compressibility, large volumes of a gas can be compressed into a small cylinder and transported easily
- Examples: liquefied petroleum gas (LPG) cylinder, Compressed natural gas (CNG) fuel tanks
- Due to high speed of particles and large space between them, gases show the property of diffusing very fast into other gases.
- Rate of diffusion is much faster than solids and liquids
- In the gaseous state, the particles move about randomly at high speed.
- Due to this random movement, they exert pressure which is the force exerted by each gas particles per unit area on the walls of the container.
3. Plasma and BEC State, Temperature
- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
Plasma and Bose Einstein condensate state
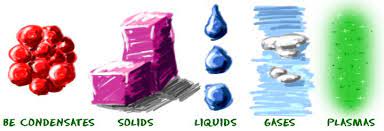
Two states of matter are plasma and BEC, let’s understand them:
Plasma: It consists of ionized gas, such that its particles are super energetic and super excited. In devices such as tube lights and CFL, the gases get ionized on the passage of current and glow with the color depending upon the nature of the gas. For Example: Neon gas emits red glow, argon emits green – yellow, etc.
Bose Einstein Condensate State: It is the fifth state of matter and it is obtained on super cooling the gas at almost absolute Kelvin zero temperature.
Temperature and temperature scales
You often fall sick, sometimes due to high fever. Then the first step that has to be taken is to measure temperature by using thermometer which records the temperature of the body in a given scale.
Temperature is the degree of hotness or coldness of body or We can also say that temperature measures the extent of motion of atoms. It is measured with the help of thermometer.

There are different scales on which temperature can be recorded:
Commonly used scales are Celsius and Kelvin
Celsius Scale:
- It is written as oC
- On it the lower fixed point (at which ice melts) is O oC
- The upper fixed point on it (at which water boils) is 100 oC
Kelvin Scale:
- The lower fixed point is- 273K
- The upper fixed point is 373 K
- Kelvin scale is called as absolute zero scale of temperature because on it the lowest possible temperature is zero
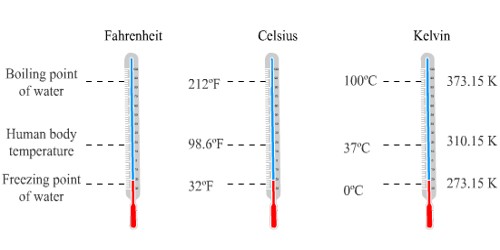
Relation between K & oC :
These scales are interconvertable by using the well defined relations like 0 oC = 273 K
So, K = oC + 273 or oC = K -273
For example, if we need to convert 25 oC to K then, we need to add 273 to it and it comes out to be 298K.
4. Interconversion of States of Matter
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
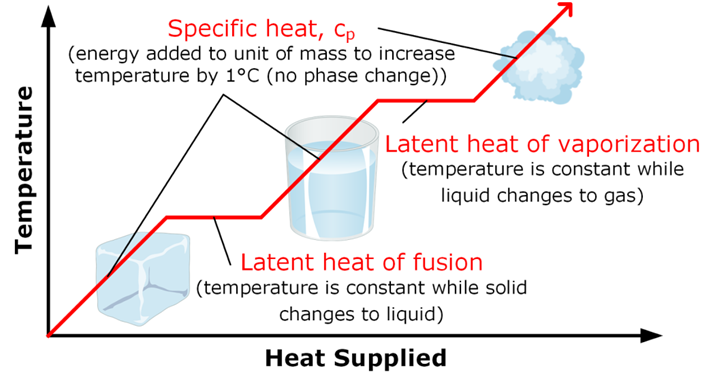
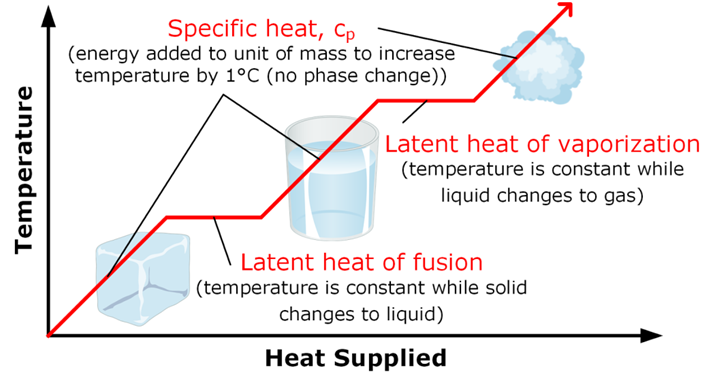
• Latent heat
The hidden heat which breaks the force of attraction between the molecules is known as the latent heat. Since, the heat energy is hidden in the bulk of the matter, it is called latent heat.
• Latent heat of fusion
→ The heat energy required to convert 1 kilogram of a solid into liquid at atmospheric pressure,at melting point, is known as the latent heat of fusion.
→ The temperature at which a liquid starts boiling, at atmospheric pressure, is called its boiling point.
• Latent heat of vaporisation
→ The heat energy required to convert 1 kilogram of liquid into gas, at atmospheric pressure, at
boiling point, is known as the latent heat of vaporisation
→ The process, in which a gas, on cooling, turns into a liquid at a specific temperature is called
condensation or liquefaction.
Formation of clouds is due to the condensation of water vapour from the Earth’s surface.
→ The temperature at which the state of a substance changes from a liquid to a solid is called freezing point of that substance.
→ When a solid melts, its temperature remains the same because heat gets used up in changing the state by overcoming the forces of attraction between the particles. It is considered that it gets hidden into the contents of the beaker and is known as the latent heat.
→ Water vapour at 373 K have more energy than water at the same temperature because particles in steam have absorbed extra energy in the form of latent heat of vaporisation.
4. Interconversion of States of Matter
CAN MATTER CHANGE ITS STATE?
Yes, they do change under various circumstances and factors which we will study below
1. Effect of change of temperature
- On increasing the temperature of solids, the kinetic energy of the particles increases.
- Due to the increase in kinetic energy, the particles start vibrating with greater speed.
- The energy supplied by heat overcomes the forces of attraction between the particles.
- The particles leave their fixed positions and start moving more freely.
- A stage is reached when the solid melts and is converted to a liquid.
- The minimum temperature at which a solid melts to become a liquid at the atmospheric pressure is called its melting point.
- The melting point of ice is 0° C (273.15 K).
- The process of melting, is also known as fusion.
- When a solid melts, its temperature remains the same
- This increase in temperature (heat) of solids is used up in changing the state by overcoming the forces of attraction between the particles without showing any rise in temperature
- This hidden heat is called latent heat
- Therefore, the amount of heat energy that is required to change 1 kg of a solid into liquid at atmospheric pressure at its melting point is known as the latent heat of fusion.
- So, particles in water at 0° C (273 K) have more energy as compared to particles in ice at the same temperature.
- Similarly, when we supply heat energy to water, particles start moving faster.
- At a certain temperature, a point is reached when the particles have enough energy to break free from the forces of attraction of each other.
- At this temperature the liquid starts changing into gas.
- The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point.
- Boiling is a bulk phenomenon i.e. each particles of the liquid gain enough energy to change into the vapour state.
- For water this temperature is 373 K (100°C = 273 + 100 = 373 K).
- The input energy required to change the state from liquid to vapor at constant temperature is called the latent heat of vaporization
- Water vapour at 373 K (100° C) have more energy than water at the same temperature. This is because particles in steam have absorbed extra energy in the form of latent heat of vaporization.
- A change of state directly from solid to gas without changing into liquid state is called sublimation. Example, vaporization of camphor
- The direct change of gas to solid without changing into liquid is called deposition. Example, soot in the chimney, making dry ice (solid carbon dioxide).
2. Effect of change of pressure
- Applying pressure and reducing temperature can liquefy gases. Examples, LPG, liquid nitrogen.
4. Interconversion of States of Matter
- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
Interconversion of States of Matter
You must have noticed that if we keep ice out in kitchen, with time it gets converted into water or when water is heating and you forget to turn the gas knob off, then it keeps boiling and a stage is reached when the vessel gets empty and no water is seen inside. This is due to interconversion of states of matter.
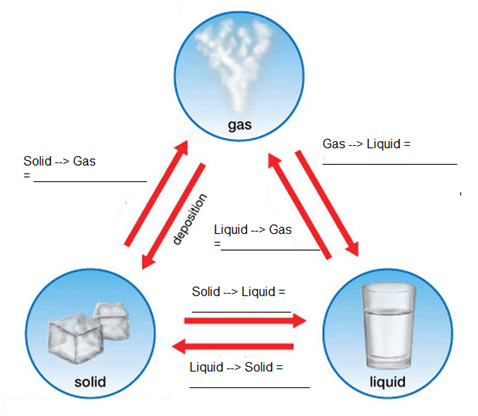
The phenomenon due to which matter changes from one state to another and then back to its original state on altering the conditions of temperature and pressure is called interconversion of states of matter.
The state of the substance can also be changed by heating or by applying pressure.
As you all have LPG cylinders at home or must have seen CNG cylinders installed in cars, these cylinders contain Liquefied Petroleum Gas or Compressed Natural Gas which means that in these cylinders the gases are liquefied by applying pressure. So, keep in mind that on applying pressure the gas can change into liquid. Similarly, on applying pressure, liquid changes into solid.
For example, when pressure is applied on Carbon Dioxide gas, it forms dry ice (Solid state). So, interconversion tells us about the change of one state of matter in to another state.
Solid ↔ liquid ↔ gas
1. The conversion of solid to liquid is called melting and it occurs at fixed temperature called melting point
2. The conversion of liquid to gas is called evaporation and it occurs at fixed temperature called as boiling point
3. The conversion of gas to liquid is called condensation or liquefaction and it occurs at liquefaction point
4. The conversion of liquid to solid is called freezing and it occurs at temperature called freezing point.
5. The conversion of solid liquid gas involves absorption of heat energy
6. And the conversion of gas liquid solid involves release of heat energy
The energy which is required to change the state whether released or absorbed is called latent heat
Latent heat
It is the amount of heat released or absorbed during change of state of a substance.
Solid liquid
Like when we convert Solid to Liquid, heat is supplied and that heat is called latent heat of fusion that is –
Latent Heat of Fusion:
it is the amount of heat required to convert one unit mass of substance from solid to liquid state at its melting point. Example: for ice it is equal to 3.34 x 105 3/Kg
Change of state of solid to liquid on the basis of molecular structure
When we heat any solid substance, the particles gain energy and start vibrating with greater amplitude. Their kinetic energy increases due to which their intermolecular space increases and intermolecular force decreases and it gets converted into liquid state.
Liquid state gaseous state
Boiling or Vaporization: it is the change of liquid state of a substance to gaseous state at boiling point. Boiling Pt. => Is the temperature at which liquid changes to gaseous state on heating. Boiling point of water => 100 oC
Latent heat of Vaporization:
it is the amount of heat required to convert one unit mass of substance from liquid to vapour at its boiling point. For example, for H2O it is equal to 22.6 x 105 J/kg.
Change of state of liquid state to gaseous state on the basis of molecular structure
When we heat any liquid substance, the particles gain energy. As a result, their kinetic energy increases and they starts vibrating with greater amplitude due to which their intermolecular space increases and intermolecular force decreases and they get converted into gaseous state.
Gas Liquid
Condensation: It is the change of gaseous state to liquid state on cooling. This occurs at a temperature below the boiling point of substance
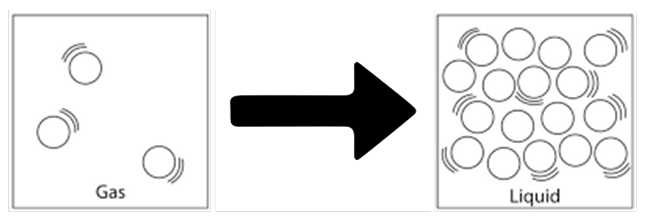
Change of state of gaseous state to liquid state on the basis of molecular structure
It is done by applying pressure and reducing temperature Explanation: When high pressure is applied on gas, distance between particles decreases & particles start attracting each other & gas gets converted in to liquid state.
Solid gas
You must have noticed that the naphthalene balls are used to prevent growth of insects in boxes of beds. With time, these balls disappear. Do you know what is the phenomenon behind this disappearance?
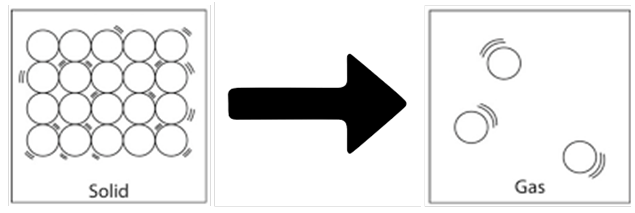
Sublimation: It is the change of solid state directly to gaseous state on heating & vapours back to solid on cooling.
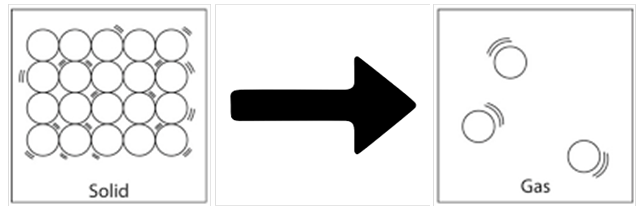
It involves two terms, that is
1. Sublime: A gaseous state that is formed due to sublimation of solid
2. Sublimate: It is the solid that sublimes
Example of Sublimation: Sublimation of Naphthalene Balls
Evaporation
You must have seen that when wet clothes are spread under sun, they dry up or the wheat grains are often dried under the Sun before sending to mill for converting to flour. This is due to the phenomenon of evaporation that is taking place.
Evaporation is the process of change of liquid into vapor at temperature below its boiling point.
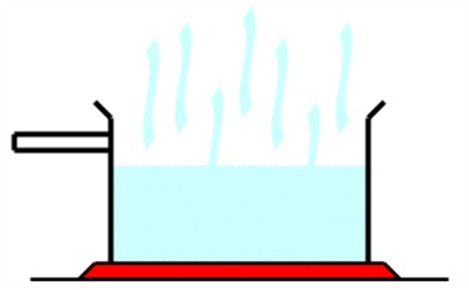
Explanation of Evaporation on the basis of Molecular Structure
During evaporation, the liquid particles are heated or they take the heat energy from the surroundings. When heat is taken by particles, the energy of particles increases due to which they start moving rapidly.
As a result, they collide with one another. In the course of collisions, some particles loose energy and some gain energy. As a result, particles with higher energy overcome their force of attraction and escape into the atmosphere.
Factors on which Evaporation Depends:
- Surface area
- Temperature
- Humidity
- Wind speed

Lets explain the dependence of the phenomenon on these given factors:
1. Surface Area – More is the exposed surface area of any substance more liquid droplets will change to vapor state For xample: Drying of Wet Clothes We have seen that clothes dry faster when they are spread on a line. The reason is that when we spread them, the surface area gets increased, therefore, evaporation gets increased as more and more molecules are exposed.
2. Temperature: Increase in temperature, increases the Kinetic Energy of molecules. They break their force of attraction, hence, escape or evaporate readily. For example: Formation of Water vapor on cup of Hot Tea.It is because as temperature rises, the particles start moving randomly, therefore, evaporation rate is increased. Therefore, when they escape, they also collide with the cup and get condensed.
3. Humidity: More is Humidity less in the Evaporation For example- Clothes dry with difficulty on a humid day: because as humidity is more, that is more vapour in the atmosphere. Due to which the escaping tendency of vapours decreases so as the rate of evaporation decreases.
4. Wind Speed: With increase in speed of wind, evaporation increases. That is the reason why clothes dry faster if there is a windy day. As the speed of wind is high, the liquid particles take up the energy. As a result, their kinetic energy increases and they escape more readily and more easily.
Evaporation Cause Cooling Effect
Evaporation is a surface phenomenon. The particles at the surface having high K.E. break up their force of attraction & form vapours. Since the energy is lost, that means temperature gets lowered and low temperature means cooling effect so, evaporation results in cooling effect
Q. Do you know why we feel more comfortable if we wear cotton clothes in summers?
A. It is because the cotton clothes are very good absorbers of Water. They rapidly absorb sweat and then evaporate taking large amount of heat from our body thereby, giving a cool sensation.
5. Evaporation
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
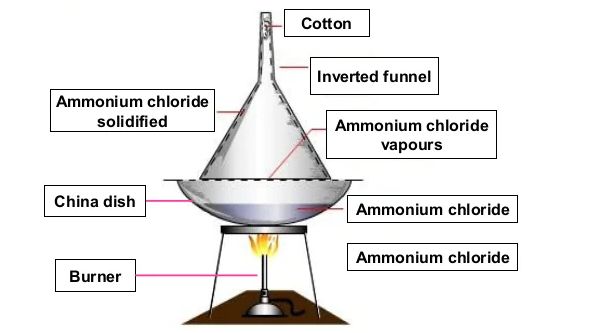
Sublimation –
→ The change of state of a substance directly from a solid to gas, without changing into the liquid
state (or vice versa) is called sublimation.
Examples – Naphthalene balls, Camphor, Ammonium chloride, iodine etc.
5. Evaporation
EVAPORATION
- In liquids, a small fraction of particles at the surface, having higher kinetic energy, is able to break away from the forces of attraction of other particles and gets converted into vapour. This phenomenon of change of a liquid into vapours at any temperature below its boiling point is called evaporation.
1.Factors affecting evaporation
- The rate of evaporation increases with
- increase of surface area
- increase of temperature
- decrease in humidity
- increase in wind speed
1. How does evaporation cause cooling?
The particles of liquid absorb energy from the surrounding to regain the energy lost during evaporation. This absorption of energy from the surroundings makes the surroundings cold.
-Why should we wear cotton clothes in summer?
* During summer, we perspire more because of the mechanism of our body which keeps us cool. We know that during evaporation, the particles at the surface of the liquid gain energy from the surroundings or body surface and change into vapour. The heat energy equal to the latent heat of vaporisation is absorbed from the body leaving the body cool. Cotton, being a good absorber of water helps in absorbing the sweat and exposing it to the atmosphere for easy evaporation.
-Why do we see water droplets on the outer surface of a glass containing ice-cold water?
* If we take some ice-cold water in a tumbler. Soon water droplets forms on the outer surface of the tumbler. The water vapour present in air, on coming in contact with the cold glass of water, loses energy and gets converted to liquid state, which we see as water droplets.
EFFECT OF CHANGE OF PRESSURE
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Effect of change of pressure
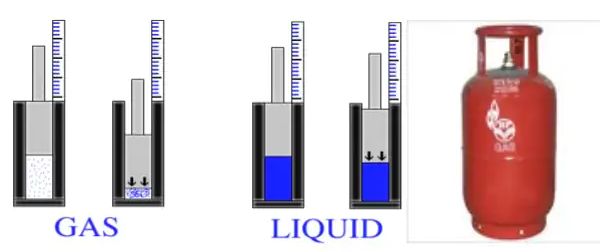
→ Gases can be liquefied by applying pressure and reducing the temperature. When a high pressure is applied to a gas, it gets compressed and if the temperature is lowered, the gas is liquefied.
→ Solid CO2 gets converted directly to gaseous state on decrease of pressure to 1 atmosphere without coming into liquid state. This is the reason that solid carbon dioxide is also known as dry ice
EVAPORATION
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Evaporation
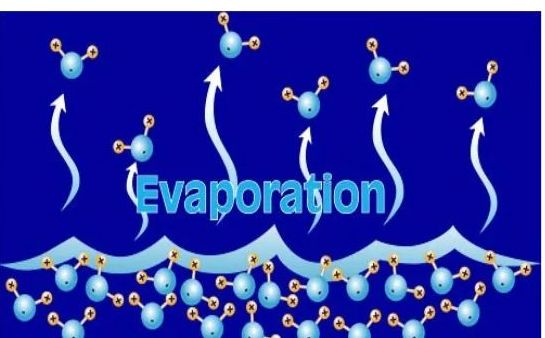
→ The process of conversion of a substance from the liquid state to the gaseous state at any temperature below its boiling point is called evaporation or vaporisation.
→ Evaporation is a surface phenomenon.
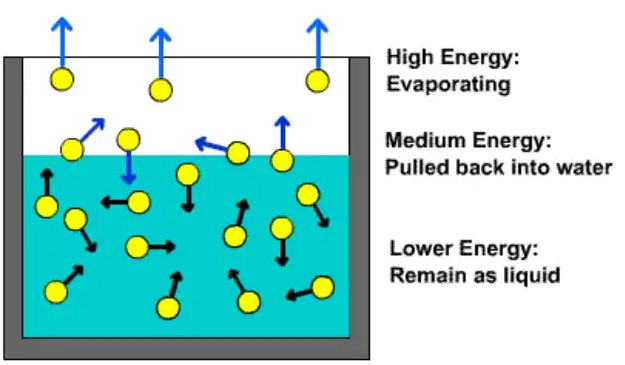
• Factors affecting the rate of evaporation
→ Surface Area – The rate of evaporation increases on increasing the surface area of the liquid.
→ Temperature - The rate of evaporation increases with an increase in temperature.
→ Humidity - Decrease in the humidity increases the rate of evaporation.
→ Wind Speed - An increase in the wind speed increases the rate of evaporation.
• Evaporation causes cooling
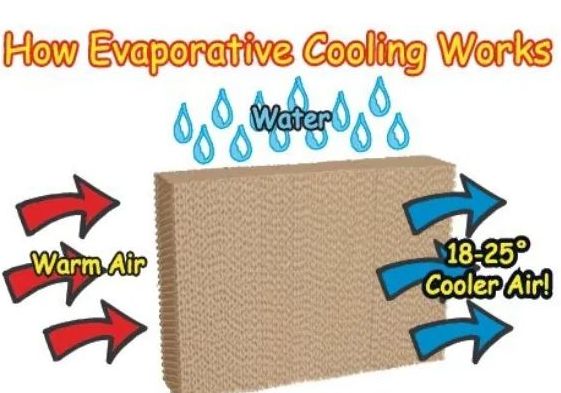
→ The particles of liquid absorb energy from the surrounding to regain the energy lost during
evaporation. This absorption of energy from the surroundings make the surroundings cold.
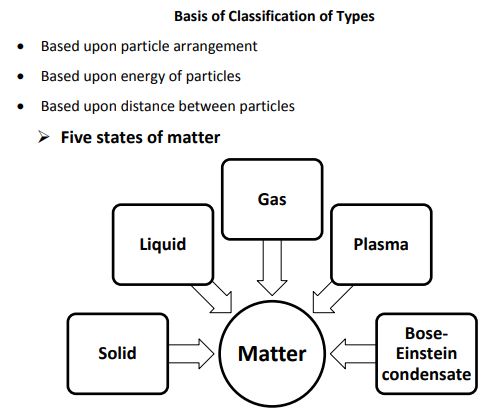
→ Lately, scientists are talking about five states of matter or five phases of matter. These are-so
liquids, gases, plasmas and the Bose–Einstein condensate.
OTHERS STATES OF MATTER
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Plasma
The state consists of super energetic and super excited particles. These particles are in the form
ionised gases. The fluorescent tube and neon sign bulbs consist of plasma.
Bose-Einstein Condensate
→ Indian physicist Satyendra Nath Bose made a study regarding the fifth state of matter. Base
his study, Albert Einstein predicted a fifth state of matter called the Bose-Einstein Condensate.
→ The Bose-Einstein Condensate or BEC is formed by cooling a gas of extremely low density to
low temperatures.
Conversion of Temperature –
→ Kelvin is the SI unit of temperature, C = 273.16
For convenience, we take 0° C = 273 K
after rounding off the decimal. To change a temperature
on the Kelvin scale to the Celsius scale you have to subtract 273 from the given temperature, and to convert a temperature on the
Celsius scale to the Kelvin scale you have to add 273 to the given temperature.
→ Atmosphere (atm) is a unit of measuring pressure exerted by a gas. The unit of pressure is Pascal.
(Pa): 1 atmosphere = 101325 Pa.
The pressure of air in atmosphere is called atmospheric pressure & atmospheric pressure at sea level is 1 atmosphere, and is taken as the normal atmospheric
pressure.
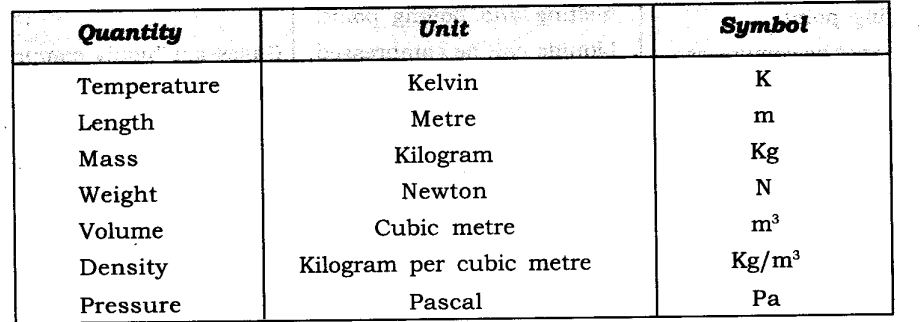
Some measurable quantities and its units

 EduMple Learning
EduMple Learning
 ACERISE INDIA
ACERISE INDIA
