- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Atomic Models
→ From the knowledge of existence of subatomic particles like electron, proton and neutron in
atom, various atomic models were proposed by different scientists.
• Some of the atomic models:
(i) Thomson’s Model of Atom
(ii) Rutherford’s Model of Atom
(iii) Bohr’s Model of Atom
→ The most trusted and scientifically established model of atom which is adopted these days
‘Quantum Mechanical Model of Atom’. It will be dealt in higher classes.
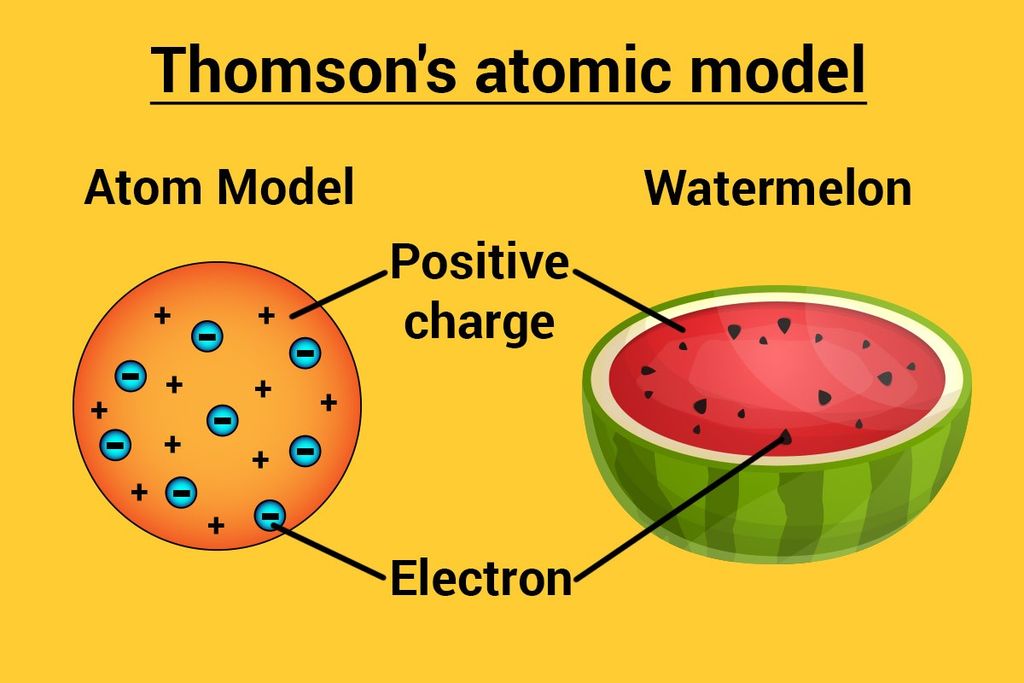
Thomson’s Atomic Model
→ This model is often called the ‘Water Melon Model’.
→ In this model, Thomson predicted the presence of electrons inside positive sphere (made up
protons), just same as seeds of watermelon are embedded in red edible part of watermelon.
→ Although this model explained neutrality of atom but couldn’t able to explain other scientific
experiments conducted on atom. Hence it was discarded.
Valency
• Valence Electrons – Electrons existing in the outermost orbit of an atom are called Valence Electrons.
• The atoms which have completely filled the outermost shell are not very active chemically.
• The valency of an atom or the combining capacity of an atom is given by the number of elements present in the outermost shell.
• For Example, Helium contains two electrons in its outermost shell which means its valency is two. In other words, it can share two electrons to form a chemical bond with another element.
• What happens when the outermost shell contains a number of electrons that are close to its maximum capacity?
Valency in such cases is generated by subtracting the number of electrons present in the outermost orbit from octet (8). For example, oxygen contains 6 electrons in its outermost shell. Its valency is calculated as: 8 – 6 = 2. This means oxygen needs two electrons to form a bond with another element.
Atomic Number of an Element
Atomic Number (Z) = Number of protons in an atom
Mass Number of an Element
Mass Number = Number of protons + Number of neutrons
Isotopes
• The atoms of an element can exist in several forms having similar atomic numbers but varying mass numbers.
• Isotopes are pure substances.
• Isotopes have a similar chemical nature.
• Isotopes have distinct physical characteristics.
Where can we use Isotopes?
1. The fuel of Nuclear Reactor – Isotope of Uranium
2. Treatment of Cancer – Isotope of Cobalt
3. Treatment of Goitre – Isotope of Iodine
Isobars
The atoms of several elements can have a similar mass number but distinct atomic masses. Such elements are called Isobars.
Isotones
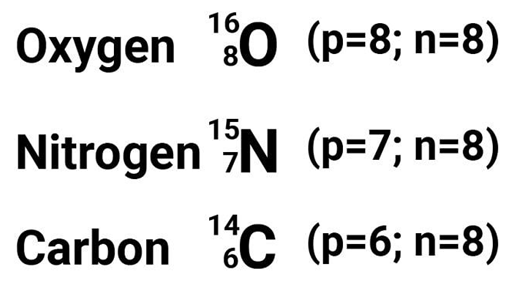
• Species having same number of neutrons but different number of protons are called Isotones.
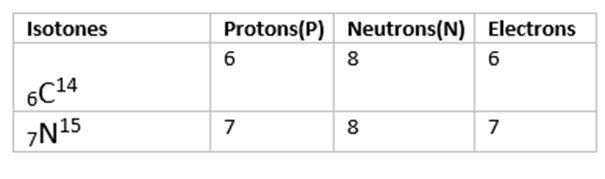
• Examples include boron-12 and carbon-13 nuclei both contain 7 neutrons, and so are isotones.
Difference Between Isotopes, Isobars & Isotones

- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
Some Important Defintion
Atomic number (z): Is defined as “Number of protons in atom”.
Mass number (A): Is defined as “sum of protons and neutrons present in the nucleus of an atom.”
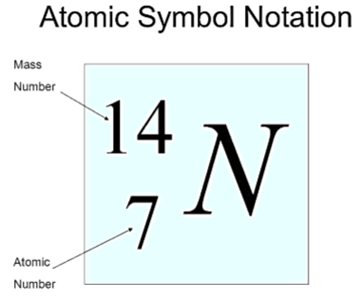
Representation of an element
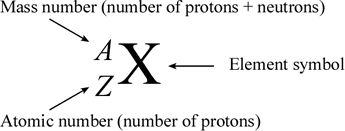
Where Z = atomic number (Sub script)
Where A= mass number (Superscript)
Electronic configuration It is defined as “Arrangement of electrons in different shells.” The arrangement is according to Bohr bury rule that is 2n².
Valence shell It is the last shell of an atom. For example, in the case of Sodium, the electronic configuration is 2,8, 1. In this, the shells involved are K,L,M. Therefore, the last shell is M (valence shell).
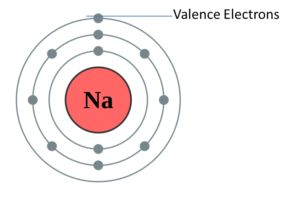
Valence electrons The electrons present in the valence shell of an atom, for example, in the case of Sodium, the electronic configuration is 2,8, 1. In this, the shells involved are K,L,M. The electrons present in valence shell are valence electrons that are 1.
Valency It is the combining capacity of an atom. It depends upon the number of valence shell electrons that is electrons in the last shell of an atom (that can be seen while writing electronic configuration). We have two types of valency that is:
- Electro-valency
- Covalence
- Electro-valency: It is the valency that is attained by losing and gaining of electrons. For example, in the case of Sodium, the atomic number is 11 and electron distribution is 2,8,1. So, in order to attain stability, it can either lose one electron or gain 7 electrons. Out of the two oprions, losing 1 electron is easier, therefore, it loses one electron and therefore, its valency is +1.
- Covalence: It is the valency that is attained by sharing of electrons. For example, in the case of carbon, it can attain stability by sharing 4 electrons. If we look at its distribution, it needs four more electrons, so it completes its octet by sharing four electrons. Therefore, its valency is -4.
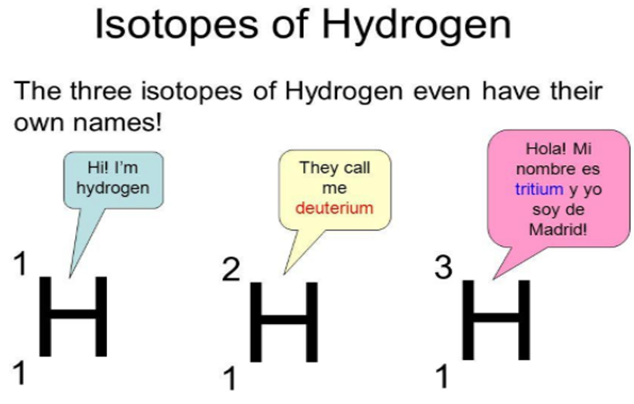
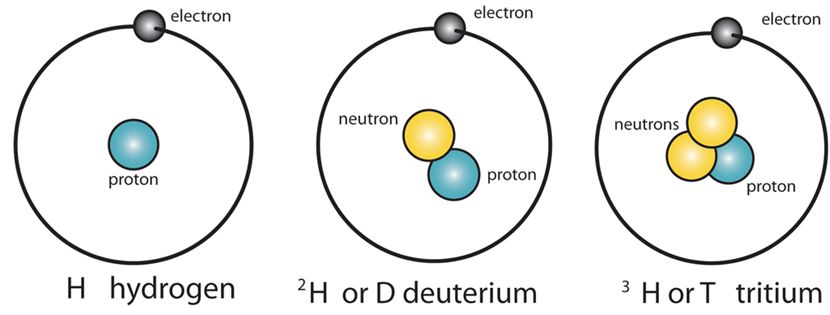
Isotopes
They are those elements which have the same atomic number but different mass numbers.
Properties of isotopes are as follows
- The number of protons is the same.
- The symbol is the same.
- Number of valence electrons and valency is same.
- They differ only in number of neutrons.
Applications of isotopes
- U-235 is used as a nuclear fuel.
- Co-60 used in the treatment of cancer.
- I-128 used in the treatment of goitre.
- C-14 is used in carbon dating.
For example:
- In the case of Hydrogen, the isotopes are – H1 H2 H3 . (But atomic number for all is 1).
- In case of oxygen, the isotopes are – O16 O17 O18 . (But atomic number for all oxygen is 8).
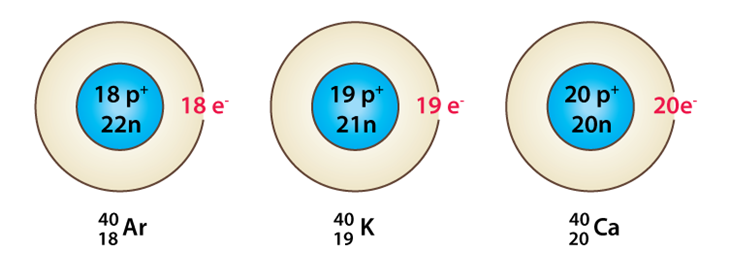
Isobars
They are those elements which have the same mass number but different atomic number. For example: sodium and magnesium are isobars as they have different atomic numbers but same mass number that is 24.
Isotones
They are those elements which have the same number of neutrons. For example: carbon, nitrogen and oxygen all have 8 neutrons.
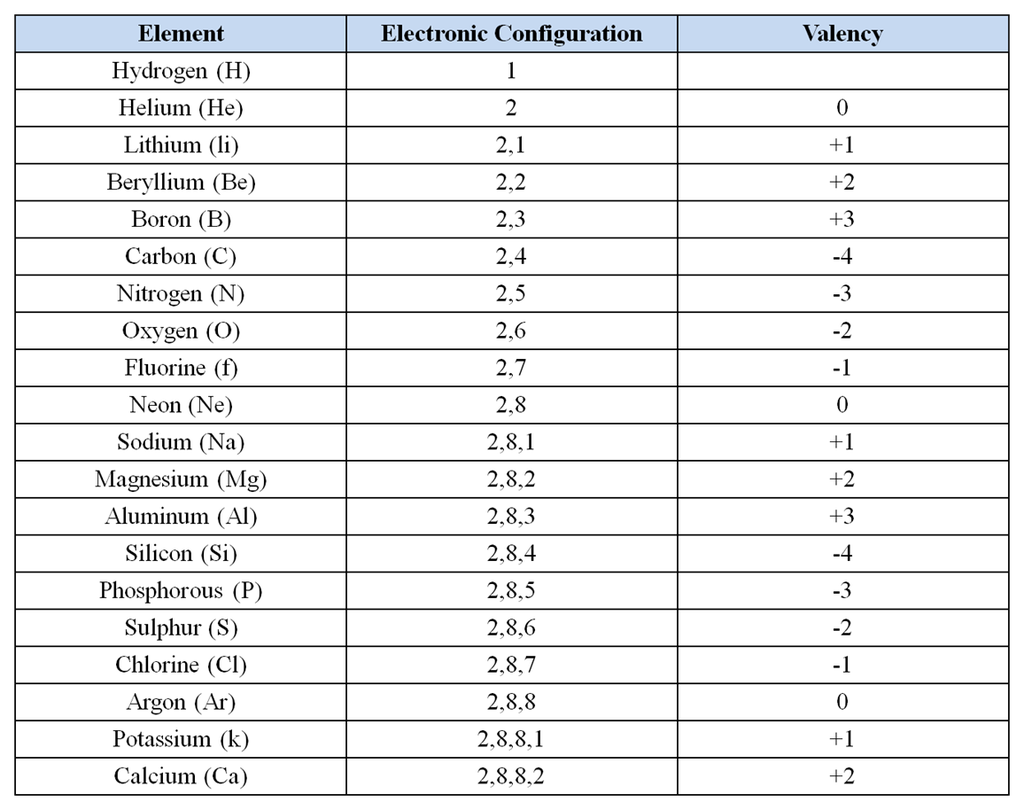
The atomic numbers of various elements along with electronic configuration and valency is given below
Atomic numbers from 1 to 20 are as follows

 Science Made Easy
Science Made Easy
 ACERISE INDIA
ACERISE INDIA
