PHYSICAL NATURE OF MATTER
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Physical Nature of Matter
→ Matter is made up of particles. All matter constitute of very small particles. These small particles are called atoms.
→ These particles of matter are too small so they cannot be seen by naked eyes or simple microscope.
→ Particles of matter are continuously moving as they posses kinetic energy, with the increase in temperature the kinetic energy of particles also increases so particle moves faster.
1. Matter and its Classification
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
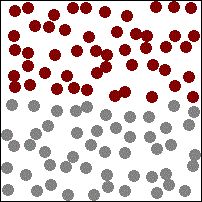
Diffusion
→ Brownian Motion: The zig-zag or random path travelled by the particles of matter is called Brownian motion.
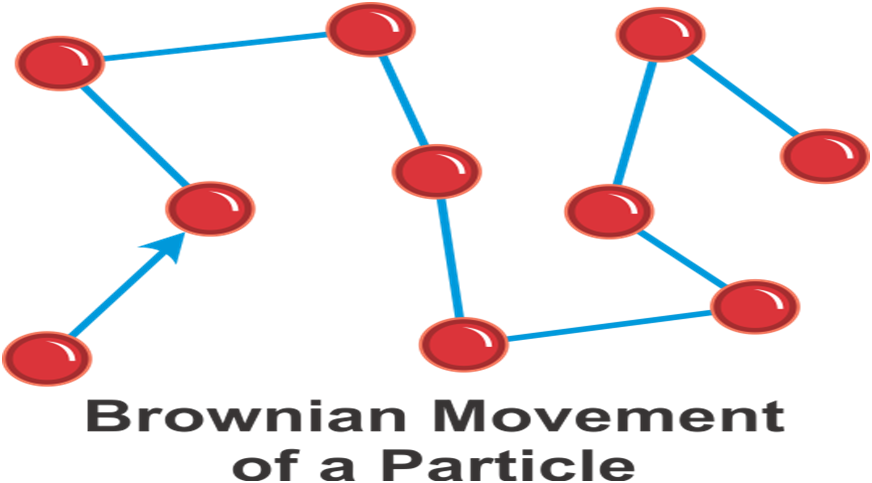
→ Intermixing of particles of two different types of matter on their own is called diffusion.
The rate of diffusion increases on increasing the temperature of the diffusing substance (by heating).
Example - The colour of ink spreading in water due to diffusion of particles of water.

2. Molecular Theory of Matter
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Characteristics of Particles of Matter
• Particles of matter have space between them
→ Gas can be compressed a lot because of the space between their particles.
Activity:- take some water in a beaker and note its level. Dissolve some salt or sugar in it with the help of a glass rod. The salt dissolves in the water but the level of water dose not change. This is because the particles of salt get the space between the particles of water.
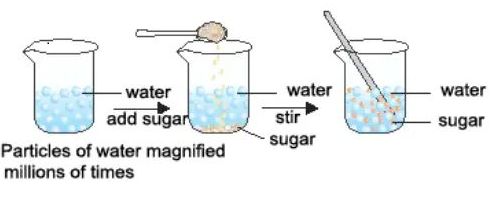
→ Additionally you would notice that there is no rise of water level takes place when one or two teaspoon of sugar/salt is added into a glass of water, this is because sugar/salt particles get adjusted in the space between the particles of water and no rise in the water level comes in result.
• Particles of matter attract each other because of force of attraction.
Activity:- Take an iron nail, a piece of chalk and rubber band. Try breaking them hummering, cutting or stretching. It is more easier to break the chalk, less easier to break the rubber band and difficult to break the iron nail. this is because the particles in the iron nail are held together with greater force than in the rubber band or challk.
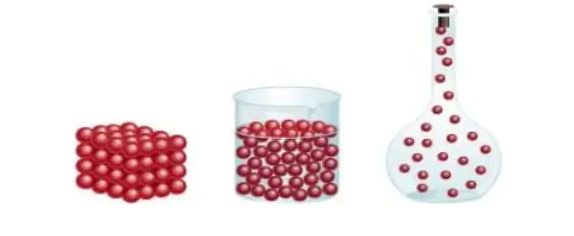
→ Force of attraction between particles of matter keeps the particles bonded together. Therefore attraction between particles of solid is greatest, between particles of liquid is moderate and between particles of gas is lowest.
→ Because of the lowest force of attraction between the particles of gas we can move our hand through
air easily. To move our hand in liquid, such as water, we have to apply some force, but a solid such as wood, we cannot move our hand.
→This is because the force of attraction between particles of gas is almost negligible, in liquid the forces of attraction is moderate but it is greatest in solid.
→ The force of attraction between particles of solid, liquid and gas can be arranged in decreases in order as follows:
Solid > Liquid > Gas
• Particles of matter are continuously moving –
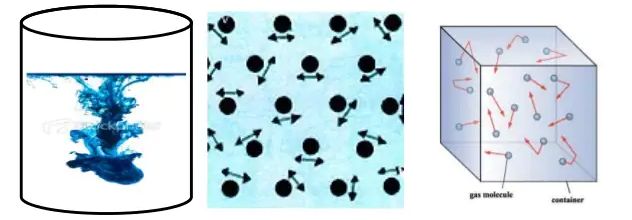
Activity :- Take some water in a beaker and put a drop of blue or red ink slowly along the sides of the beaker. Leave it undisturbed for a few hours. The ink spreads evenly throughout the water due to the movement of the particles of water and ink.
The intermixing of two or more different types of matter on their own is called diffusion.
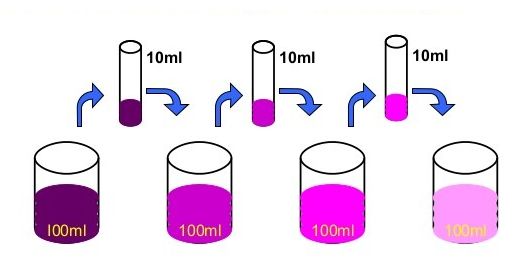
• Matter is made up of small particles –
Activity :- Dissolve 2 —3 crystals of potassium permanganate in I OOml of water in a beaker. Take lOmI of this solution and dissolve in lOOmI of water. Take lOmI of this solution and dissolve In lOOmI of water. Repeat this process 5— 6 times. This shows that a few crystals of potassium permanganate can colour a large volume of water because there are millions of tiny particles in each crystal
Classification of states of matter
States of Matter
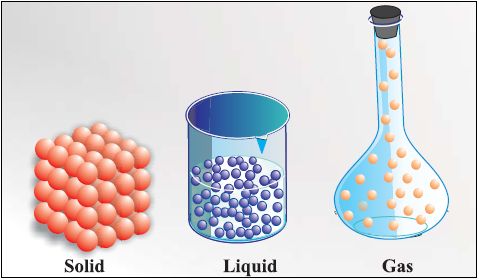
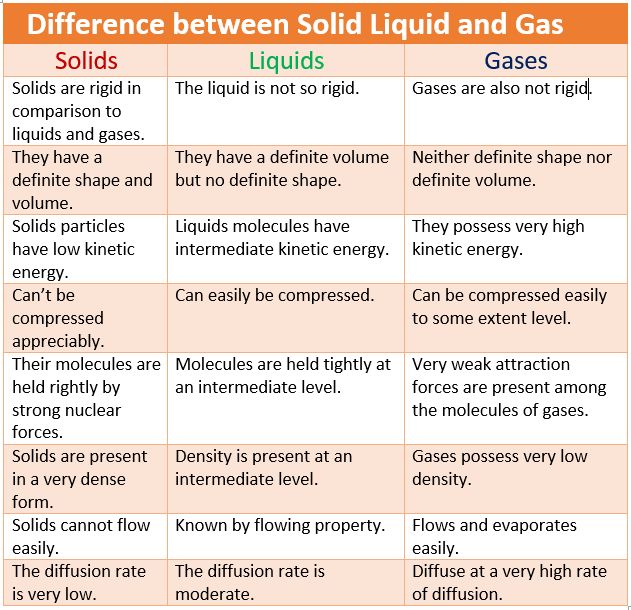
• Solid State
→ The space between the particles is very less.
→ The force of attraction between the particles is strong. Thus, particles in a solid are closely packed.
→ Solids maintain their shape even when they are subjected to external force i.e. they are rigid
→ Solids cannot be compressed.
→ The kinetic energy of the particles is very less and so solids have an orderly arrangement of particles. Therefore, solids have a fixed shape and volume.
• Liquid State
→ The space between the particles is slightly more as compared to solids, but still very less as compared to gases. The particles of a liquid can slip and slide over each other.
→ The force of attraction between the particles is strong enough to hold the particles together not strong enough to hold the particles in a fixed position.
→ Liquids do not have a fixed shape but have a fixed volume. Liquids take up the shape of the container in which they are poured.
→ The kinetic energy of the particles is more than that of solids. Thus, liquids have a disorderly arrangement of particles compared solids.
→ Liquids cannot be compressed much. The compressibility of liquids is almost negligible.
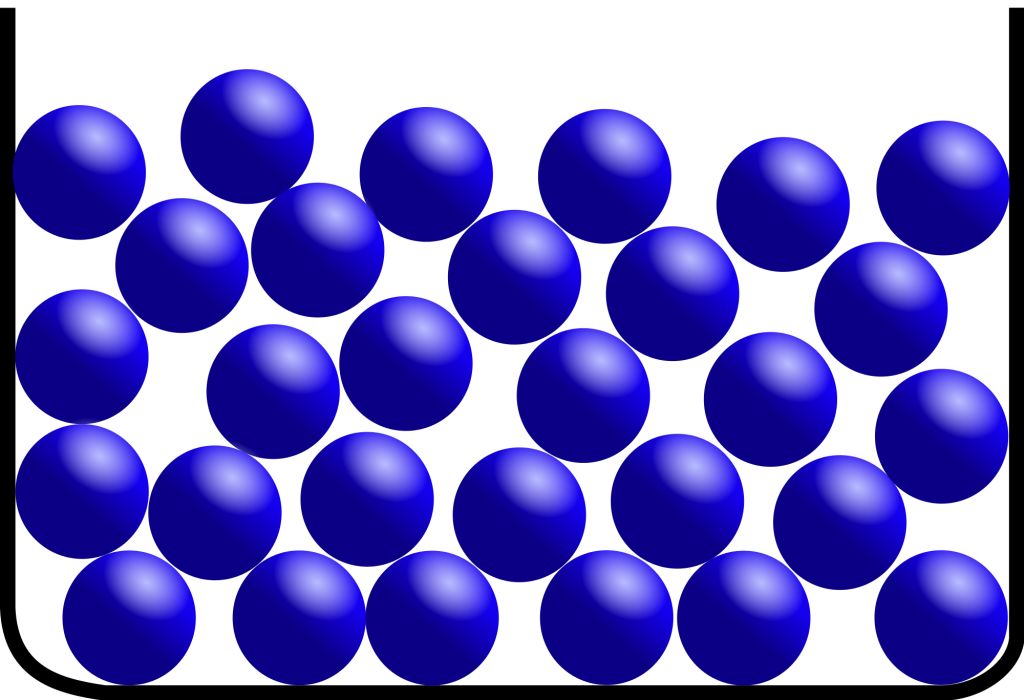
• Gaseous State
→ The particles are much farther apart from one another as compared to solids and liquids.They have a very disorderly arrangement of particles compared to the solids and liquids.
→ The force of attraction between the particles is negligible, hence particles of a gas move free in all the directions.Gases thus can mix or diffuse into other gases.
→ The particles of a gas have maximum kinetic energy. They move with high speed in all directions and can exert pressure on the walls of its container.
→ Gases neither have a definite shape nor a definite volume.They fill up the container completely
→ Gases can be compressed easily. Example: the LPG cylinders used at home and the CNG cylinder used in vehicles.
3. Plasma and BEC State, Temperature
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Change of State of Matter

→ The phenomenon of change from one state of matter to another, and then back to the original state is called the interconversion of states of matter.
→ Matter Can Change its State. Water can exist in three states of matter:
• Solid as ice
• Liquid as water
• Gas as water vapour
Effect of Temperature
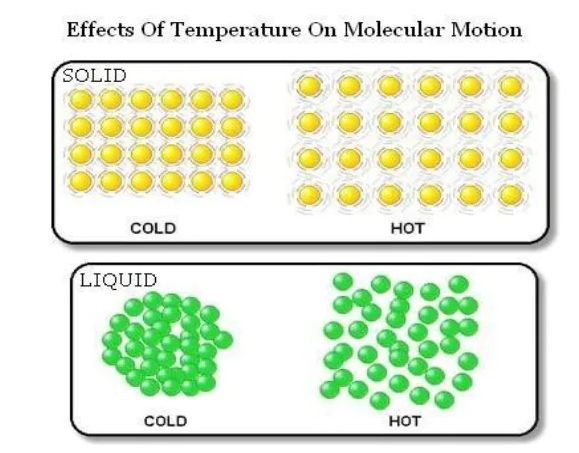
→ On increasing the temperature of solids, the kinetic energy of the particles increases which overcomes the forces of attraction between the particles thereby solid melts and is converted into liquid.
→ The temperature at which a solid melts to become a liquid at the atmospheric pressure is called its
melting point.
The melting point of ice is 273.16 K.
The process of melting, that is, change of solid state into liquid state is also known as fusion.
4. Interconversion of States of Matter
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
• Latent heat
The hidden heat which breaks the force of attraction between the molecules is known as the latent heat. Since, the heat energy is hidden in the bulk of the matter, it is called latent heat.
• Latent heat of fusion
→ The heat energy required to convert 1 kilogram of a solid into liquid at atmospheric pressure,at melting point, is known as the latent heat of fusion.
→ The temperature at which a liquid starts boiling, at atmospheric pressure, is called its boiling point.
• Latent heat of vaporisation
→ The heat energy required to convert 1 kilogram of liquid into gas, at atmospheric pressure, at
boiling point, is known as the latent heat of vaporisation
→ The process, in which a gas, on cooling, turns into a liquid at a specific temperature is called
condensation or liquefaction.
Formation of clouds is due to the condensation of water vapour from the Earth’s surface.
→ The temperature at which the state of a substance changes from a liquid to a solid is called freezing point of that substance.
→ When a solid melts, its temperature remains the same because heat gets used up in changing the state by overcoming the forces of attraction between the particles. It is considered that it gets hidden into the contents of the beaker and is known as the latent heat.
→ Water vapour at 373 K have more energy than water at the same temperature because particles in steam have absorbed extra energy in the form of latent heat of vaporisation.
5. Evaporation
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
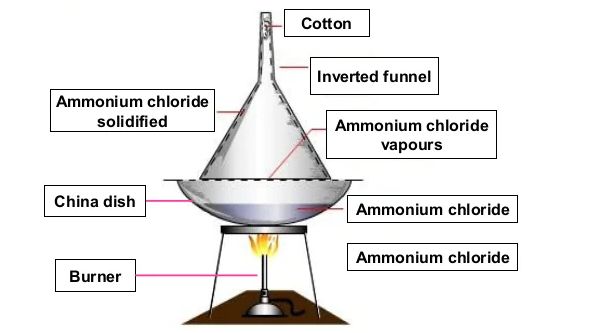
Sublimation –
→ The change of state of a substance directly from a solid to gas, without changing into the liquid
state (or vice versa) is called sublimation.
Examples – Naphthalene balls, Camphor, Ammonium chloride, iodine etc.
EFFECT OF CHANGE OF PRESSURE
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Effect of change of pressure
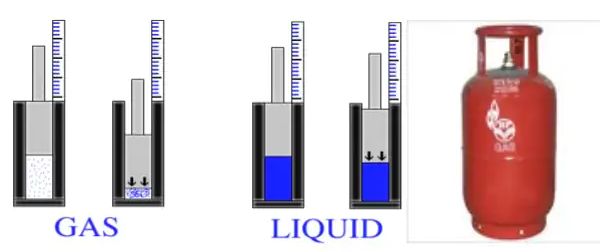
→ Gases can be liquefied by applying pressure and reducing the temperature. When a high pressure is applied to a gas, it gets compressed and if the temperature is lowered, the gas is liquefied.
→ Solid CO2 gets converted directly to gaseous state on decrease of pressure to 1 atmosphere without coming into liquid state. This is the reason that solid carbon dioxide is also known as dry ice
EVAPORATION
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Evaporation
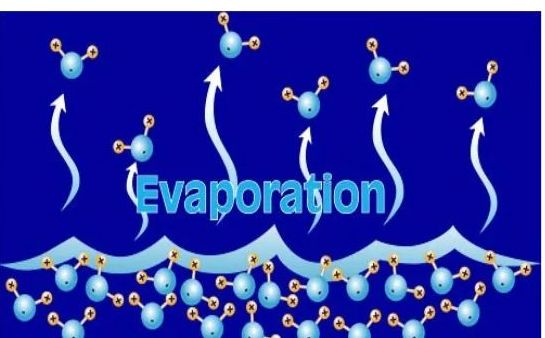
→ The process of conversion of a substance from the liquid state to the gaseous state at any temperature below its boiling point is called evaporation or vaporisation.
→ Evaporation is a surface phenomenon.
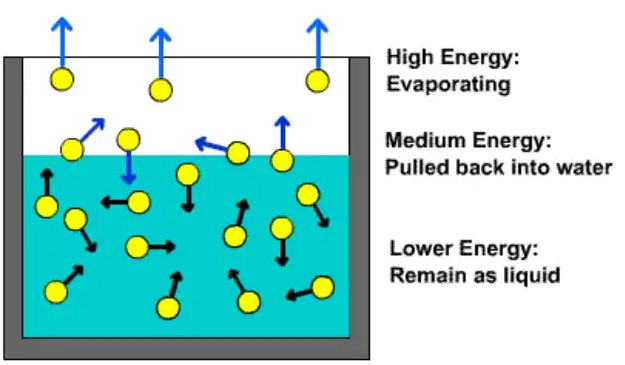
• Factors affecting the rate of evaporation
→ Surface Area – The rate of evaporation increases on increasing the surface area of the liquid.
→ Temperature - The rate of evaporation increases with an increase in temperature.
→ Humidity - Decrease in the humidity increases the rate of evaporation.
→ Wind Speed - An increase in the wind speed increases the rate of evaporation.
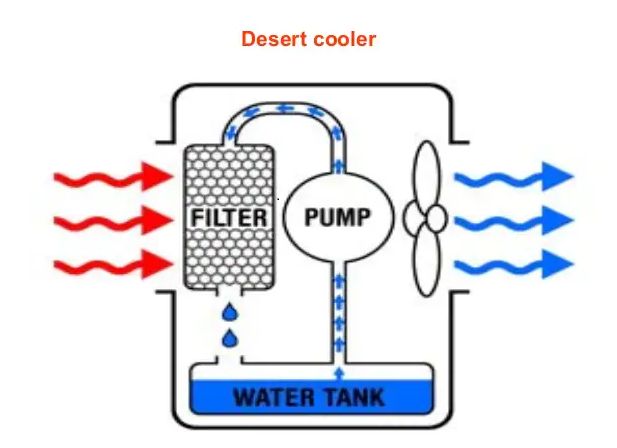
• Evaporation causes cooling
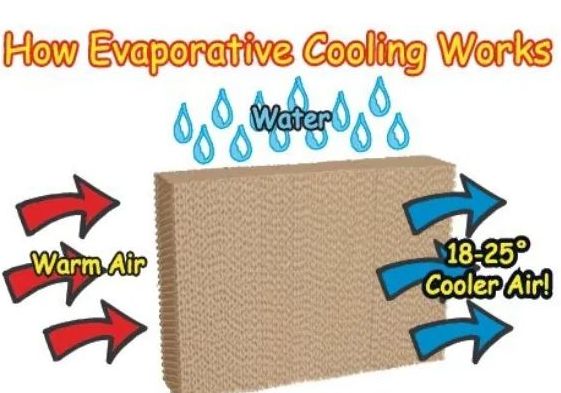
→ The particles of liquid absorb energy from the surrounding to regain the energy lost during
evaporation. This absorption of energy from the surroundings make the surroundings cold.
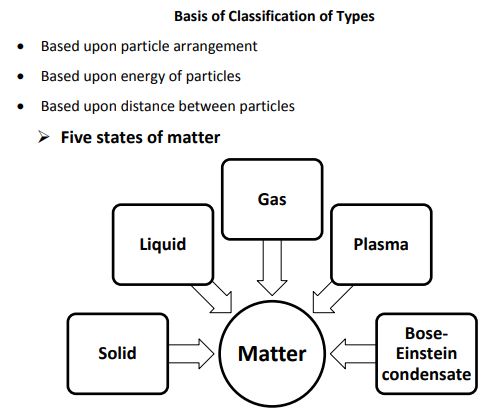
→ Lately, scientists are talking about five states of matter or five phases of matter. These are-so
liquids, gases, plasmas and the Bose–Einstein condensate.
OTHERS STATES OF MATTER
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Plasma
The state consists of super energetic and super excited particles. These particles are in the form
ionised gases. The fluorescent tube and neon sign bulbs consist of plasma.
Bose-Einstein Condensate
→ Indian physicist Satyendra Nath Bose made a study regarding the fifth state of matter. Base
his study, Albert Einstein predicted a fifth state of matter called the Bose-Einstein Condensate.
→ The Bose-Einstein Condensate or BEC is formed by cooling a gas of extremely low density to
low temperatures.
Conversion of Temperature –
→ Kelvin is the SI unit of temperature, C = 273.16
For convenience, we take 0° C = 273 K
after rounding off the decimal. To change a temperature
on the Kelvin scale to the Celsius scale you have to subtract 273 from the given temperature, and to convert a temperature on the
Celsius scale to the Kelvin scale you have to add 273 to the given temperature.
→ Atmosphere (atm) is a unit of measuring pressure exerted by a gas. The unit of pressure is Pascal.
(Pa): 1 atmosphere = 101325 Pa.
The pressure of air in atmosphere is called atmospheric pressure & atmospheric pressure at sea level is 1 atmosphere, and is taken as the normal atmospheric
pressure.
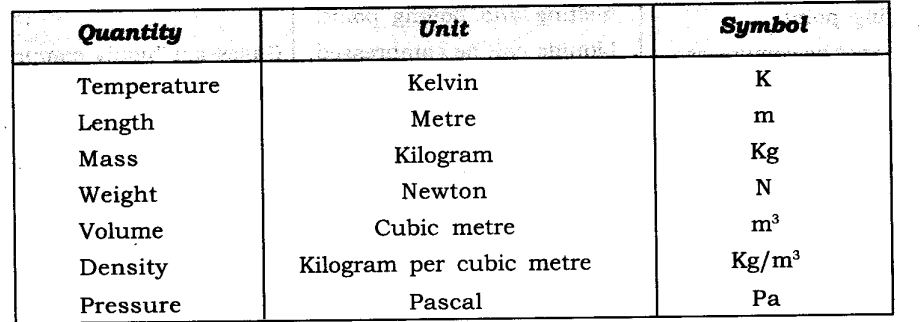
Some measurable quantities and its units
1. Pure Substance
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Introduction
Anything which occupies space and has mass is called matter.
Matter can be divided in two categories.
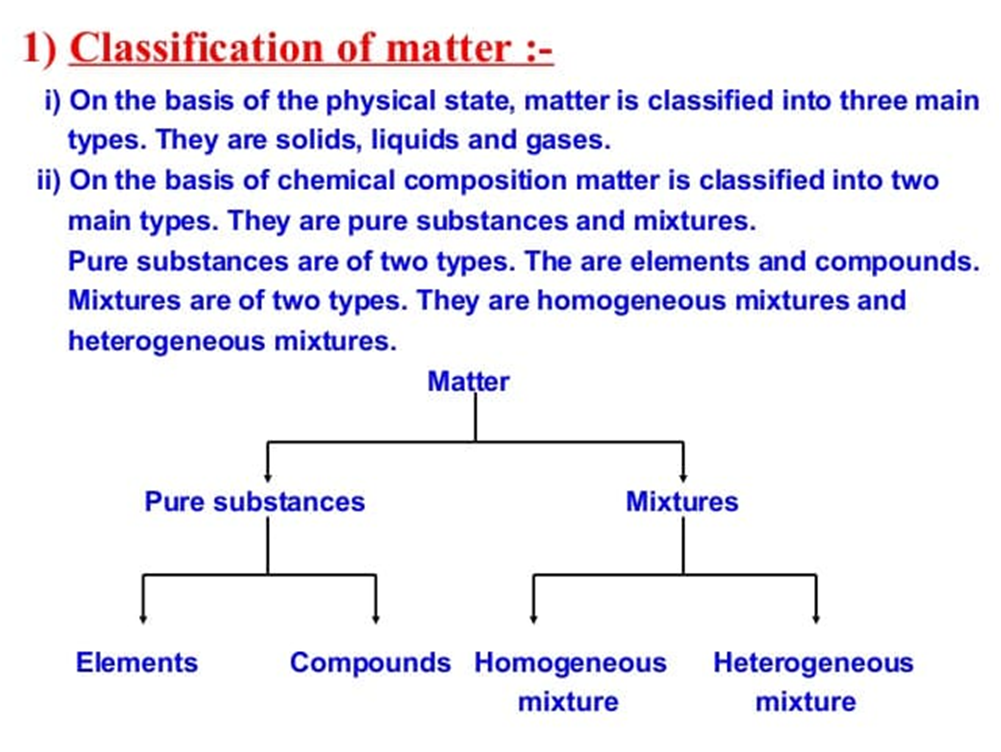
(i) Pure Substance: It consists of single types of particles which are same in their chemical nature.
(ii) Mixtures: Mixture consists of two or more particles.

Mixture and its types
Mixture consists of more than one kind of pure substances which can be separated by physical method.
Mixtures are of two types
(i) Homogeneous mixture
(ii) Heterogeneous mixture
(i) Homogeneous mixture: A mixture is said to be homogeneous if all the components of the
mixture are uniformly mixed and there are no boundaries of separation between them.
Ex: Sugar in water, etc.
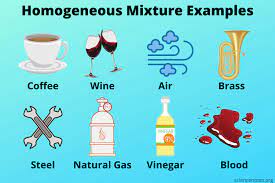
(ii) Heterogeneous mixtures: A mixture is said to be heterogeneous if all the components of the
mixture are not uniformly mixed and there are visible boundaries of separation between them
Ex: Water and sand, Air etc.

2. Impure Substance
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
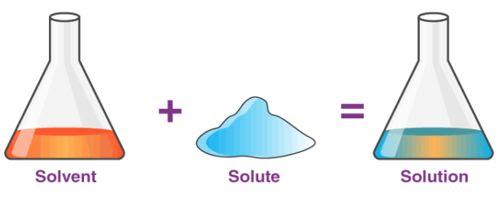
Solution and its properties
A solution is a homogeneous mixture of two or more substances. Ex: Lemonade, soda water etc
A solution has two components:
(i) Solvent
(ii) Solute
(i) Solvent: The component of the solution that dissolves the other component in it (usually the component present in larger amount) is called the solvent.
(ii) Solute: The component of the solution that is dissolved in the solvent (usually present in less quantity) is called the solute.
Properties of Solution:
1. A solution is a homogeneous mixture.
2. The particles of a solution are smaller than 1 nm
(10-9 ) in diameter which cannot be seen by naked
eyes.
3. They do not scatter a beam of light passing through the solution that is they don’t show tyndall effect. So, the path of light is not visible in a solution.
4. The solute particles cannot be separated from the mixture by the process of filtration.
5. The solution is stable and solute particles do not settle down when left undisturbed.
Concentration of a solution
(i) Saturated solution: When no more amount of solute can be dissolved in a solution at a give temperature, it is called a saturated solution.
(ii) Unsaturated solution: When more amount of solute can be dissolved in a solution at a give temperature, it is called a saturated solution.
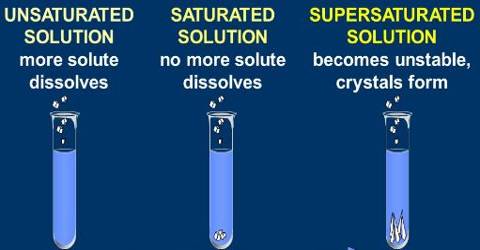
(ii) Solubility: The amount of the solute present in the saturated solution at the given temperature
called its solubility.
The concentration of a solution is the amount of solute present in a given amount (mass or volume) of solution. Also, the amount of solute dissolved in a given mass or volume of solvent is called concentration of solution.
Concentration of solution = Amount of solute/Amount of solvent or Amount of solute/Amount in
solution (Here, amount means mass or volume).
Two methods of finding concentration of solution:
(i) Mass by mass percentage of a solution = (Mass of solute/Mass of solution) ×100
(ii) Mass by volume percentage of a solution = (Mass of solute/Volume of solution) ×100
3. Separation of Mixtures
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Suspension and its properties
A suspension is a heterogeneous mixture in which the the solute particles do not dissolve but
remain suspended throughout the bulk of the medium. Ex: Chalk in water, smoke in the air.

Properties of Suspension :
1. It is a heterogeneous mixture.
2. Particles of a suspension are visible to the naked eye.
3. Size of the particles is greater than 100 nm.
4. It is unstable mixture. Solute settles down at the bottom over period of time.
5. If the solution is passed through filter paper, solute and solvent gets separated.
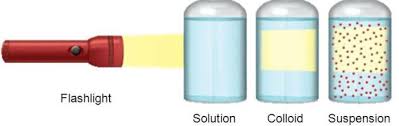
6. It scatters light when light is passed through the solution i.e. it shows Tyndall effect.
4. Physical And Chemical Changes
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Colloidal solution and its properties
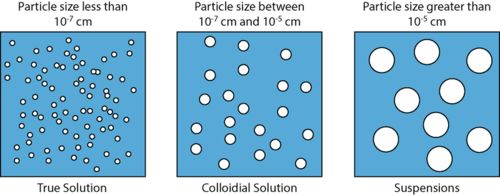
Colloid solution is heterogeneous mixture in which the size of particles lies between the true solutions and suspensions.
• Colloidal particles can easily scatter a beam of visible light. This phenomenon is called
Tyndall effect.
Properties of colloidal solution:
1. The particles of colloid can’t be seen by naked eyes individually.
2. It is a heterogeneous mixture and thus solute and solvent can’t be separated by filter paper.
3. Size of particles is smaller than suspensions but greater than solutions (1 nm to 100 nm).
4. It is a stable mixture. Particles do not settle down at the bottom over a period of time.
5. They do not settle down when left undisturbed which means colloid is quite stable.
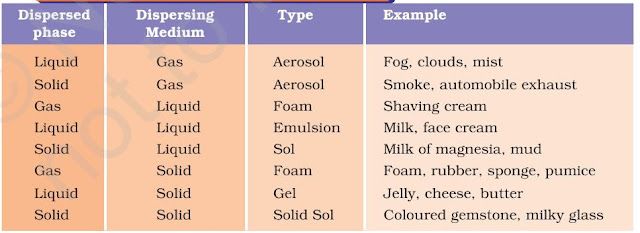
Some common examples of colloids (in the table)
5. What Are The Types Of Pure Substances ?
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Separation of the components of mixtures
Different methods of separation are used to get from mixture.
Heterogeneous mixtures can be separated into their respective constituents by simple physical
methods like handpicking, sieving, filtration etc.
Obtaining coloured components from blue/black ink
Process of evaporation is used to obtain coloured components from blue/black ink. The process of
evaporation is used to separate a substance which is dissolved in water.
• It is based on the fact that liquid vaporises easily than the solid.
• Helps in separating volatile substances from non-volatile substances.
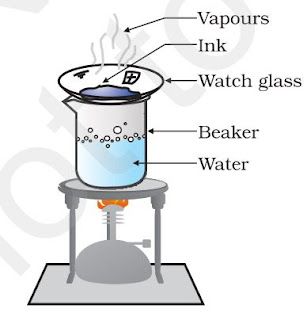
Steps of obtaining coloured components from blue/black ink:
• Fill half a beaker with water.
• Put a watch glass on the mouth of the beaker.
• Put few drops of ink on the watch glass.
• Now start heating the beaker. We do not want to heat the ink directly. You will see that evaporation
is taking place from the watch glass.
• Continue heating as the evaporation goes on and stop heating when you do not see any further
change on the watch glass.
Separation of cream from milk
• The process of centrifugation is used to separate the cream from milk. It is a method of separating
the suspended particles of substance from a liquid.
• This process is carried out by the machine called centrifuge.
• Sometimes, the solid particles in a liquid are very small and pass through a filter paper. For that
particles the filtration technique cannot be used.
• The mixture is rotated rapidly so that the heavier particles in the mixtures settle down to the
bottom.
• The basic principle of centrifugation is that the denser particles are forced to the bottom and
liquid being lighter remains at the top.
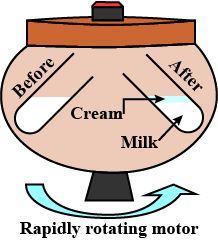
Steps of separating cream from milk:
• Take some full-cream milk in a test tube.
• Centrifuge it by using a centrifuging machine for two minutes.
Application of centrifugation:
• Used in diagnostic laboratories for blood and urine tests.
• Used in dairies and home to separate butter from cream.
• Used in washing machines to squeeze out water from wet clothes.
Separating two immiscible liquids
• The separation of separating two immiscible liquid is carried out by the use of funnel.
• The basic principle involve is the difference between the densities of two liquids form two separate layers.
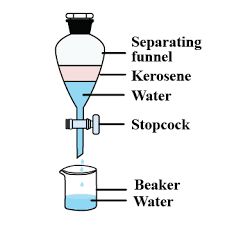
Steps of separating kerosene oil and water:
• Pour the mixture of kerosene oil and water in a separating funnel.
• Let it stand undisturbed for sometime so that separate layers of oil and water are formed.
• Open the stopcock of the separating funnel and pour out the lower layer of water carefully.
• Close the stopcock of the separating funnel as the oil reaches the stop-cock.
Application of funnel:
• To separate mixture of oil and water.
• In the extraction of iron from its ore, the lighter slag is removed from the top by this method to
leave the molten iron at the bottom in the furnace.
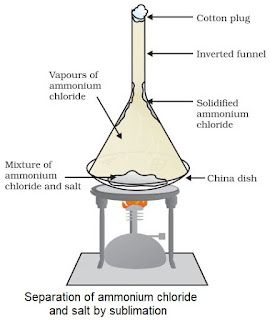
Sublimation
• This process is used to separate mixtures that contain a sublimable volatile component from
non-sublimable impurity.
• Sublimation is process where a substance directly changes from solid to gaseous state on heating.
• Ammonium chloride, camphor, naphthalene and anthracene are some examples which can be
sublime.
Chromatography
• Used to separate those solutes which dissolve in the same solvent.
• Used for sepration of colours.
• The colours which are more soluble in water rises faster.
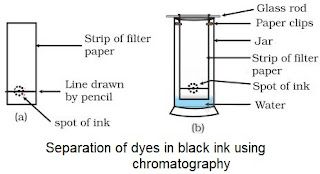
Applications
To separate
• colours in a dye
• pigments from natural colours
• drugs from blood.
Distillation
• Used for separation of components of a mixture containing two miscible liquids that boil with the
decomposition and have sufficient difference in their boiling points.
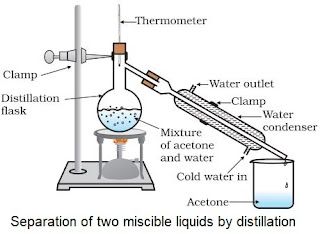
• Mixture of acetone and water is separated by this method.
Fractional distillation
• Fractional distillation is used to separate a mixture of two or more miscible liquids for which the
difference in boiling points is less than 25 K.
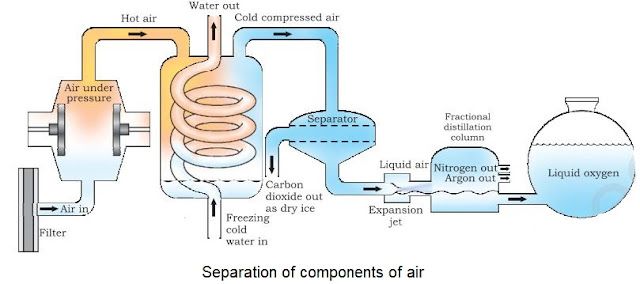
• Air is a homogeneous mixture and can be separated into its components by fractional distillation
Below is diagram which shows the steps of separation of air:
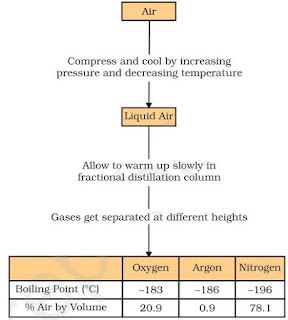
• The air is compressed by increasing the pressure and is then cooled by decreasing the temperature to get liquid air.
• The liquid air is warm-up slowly in a fractional distillation column, where gases get separated
different heights depending upon their boiling points.
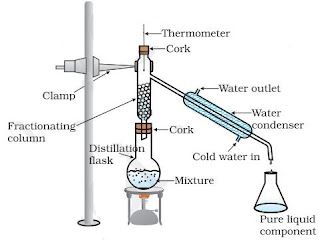
• It used to separate a gas from the air.
Crystallization
• Used to remove impurities from solid and purify it.
• It separates a pure solid from mixture in the form of crystals.
• This process is used in purification of salt from sea water, separation of crystals of alum from
impure samples.
• It is better method than evaporation because:
(i) Solids decompose or some, like sugar, may get charred on heating to dryness.
(ii) Some impurities may remain dissolved in the solution even after filtration. On evaporation the
contaminate the solid.
PHYSICAL & CHEMICAL CHANGES
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Physical and Chemical changes
• The process which brings about changes in physical properties and no new substances are formed are physical changes. The common physical changes are changes in colour, hardness,rigidity, fluidity, density, melting point, boiling point etc.
• The process in which new Free substances are formed and chemical changed are chemical changes. Some chemical properties are odour, inflammability etc.


PURE SUBSTANCES AND ITS TYPES
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Types of pure substances
The pure substance is divided in two types on the basis of their chemical composition:
(i) Elements
(ii) Compounds
(i) Elements
• According to Antoine Laurent Lavoisier, element is a basic form of matter that cannot be broken
down into simpler substances by chemical reactions.
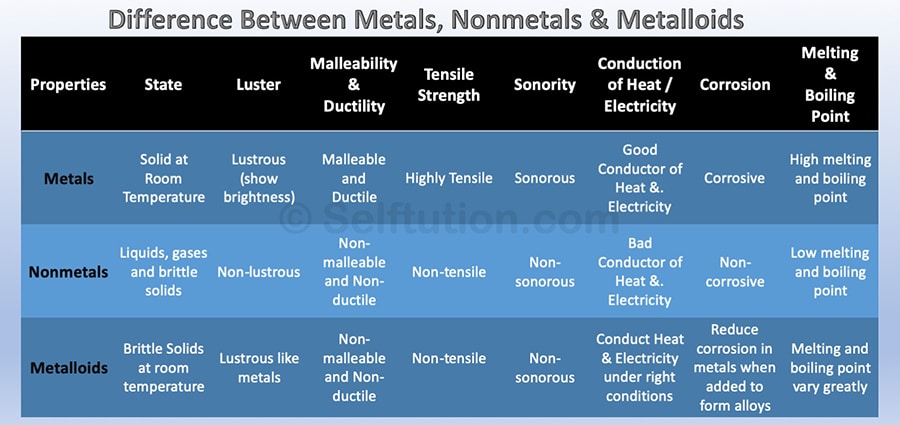
• It is divided in three types which are metals, non-metals and metalloids.
Properties of Metals
(i) They have a lustre (shine).
(ii) They have silvery-grey or golden-yellow colour.
(iii) They conduct heat and electricity.
(iv) They are ductile (can be drawn into wires).
(v) They are malleable (can be hammered into thin sheets).
(vi) They are sonorous (make a ringing sound when hit).
• Examples of metals are gold, silver, copper, iron, sodium, potassium etc.
• Mercury is the only metal that is liquid at room temperature.
Properties of non-metals
(i) They display a variety of colours.
(ii) They are poor conductors of heat and electricity.
(iii) They are not lustrous, sonorous or malleable.
• Examples of non-metals are hydrogen, oxygen, iodine, carbon (coal, coke), bromine, chlorine
Metalloids: Elements having intermediate properties between those of metals and non-metal
called metalloids. Examples are boron, silicon, germanium etc.
(ii) Compounds
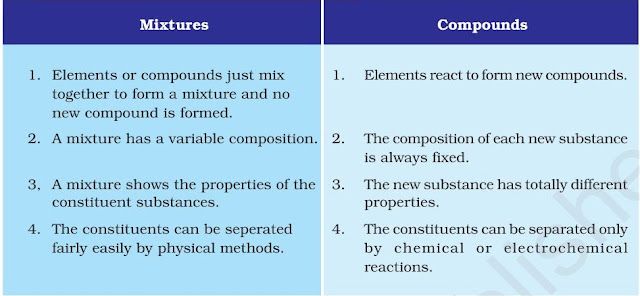
A compound is a substance composed of two or more elements, chemically combined with oneanother in a fixed proportion.
Difference between mixtures and compounds
INTRODUCTION
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Introduction
→ Around 500 B.C., Indian philosopher Maharishi Kanad, postulated the theory if we go on dividing
matter (padarth), we will obtain smallest particle beyond which further division can't be possibile
which is known as 'parmanu'.
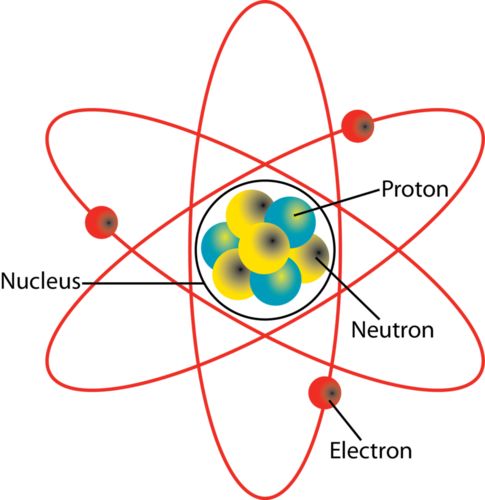
→ Ancient Greek philosophers – Democritus and Leucippus called these particles atoms.
→ Antoine L. Lavoisier laid the foundation of chemical sciences by establishing two important laws
of chemical combination.
1. Dalton's atomic Theory
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Introduction
→ Around 500 B.C., Indian philosopher Maharishi Kanad, postulated the theory if we go on dividing
matter (padarth), we will obtain smallest particle beyond which further division can't be possibile
which is known as 'parmanu'.
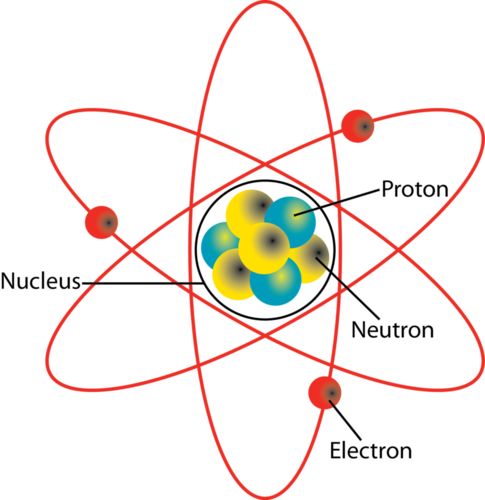
→ Ancient Greek philosophers – Democritus and Leucippus called these particles atoms.
→ Antoine L. Lavoisier laid the foundation of chemical sciences by establishing two important laws
of chemical combination.
Laws of Chemical Combination
• This law established after the experiments by Lavoisier and Joseph L. Proust.
• The chemical reaction between two or more substances give rise to products which is governed
by certain laws called Laws of Chemical Combination.
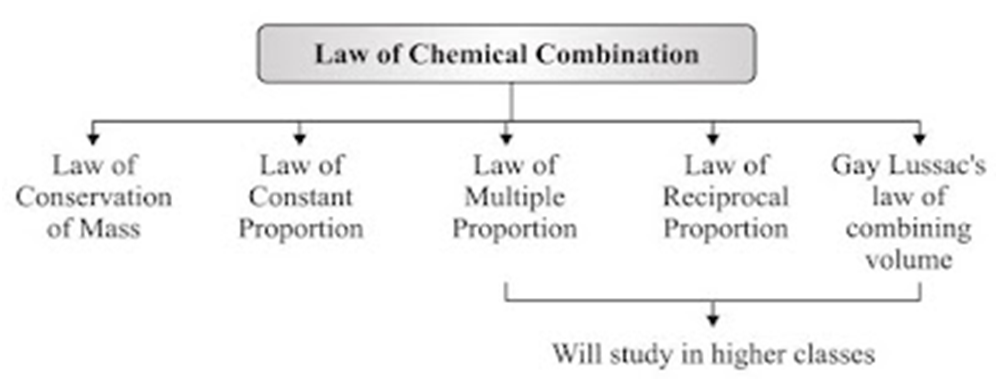
• Law of Conservation of Mass
→ During a chemical reaction, the total mass of reactants will be equal to the total mass of the products.
→ Mass can neither be created nor destroyed in a chemical reaction.
→ Example: A (reactant) + B (reactant) → AB (product)
mass of A + mass of B = mass of AB
• Law of Constant Proportions
→ In a chemical reaction, compounds always contain the same elements present in definite proportions by mass irrespective of their source.
→ It was given by Lavoisier.
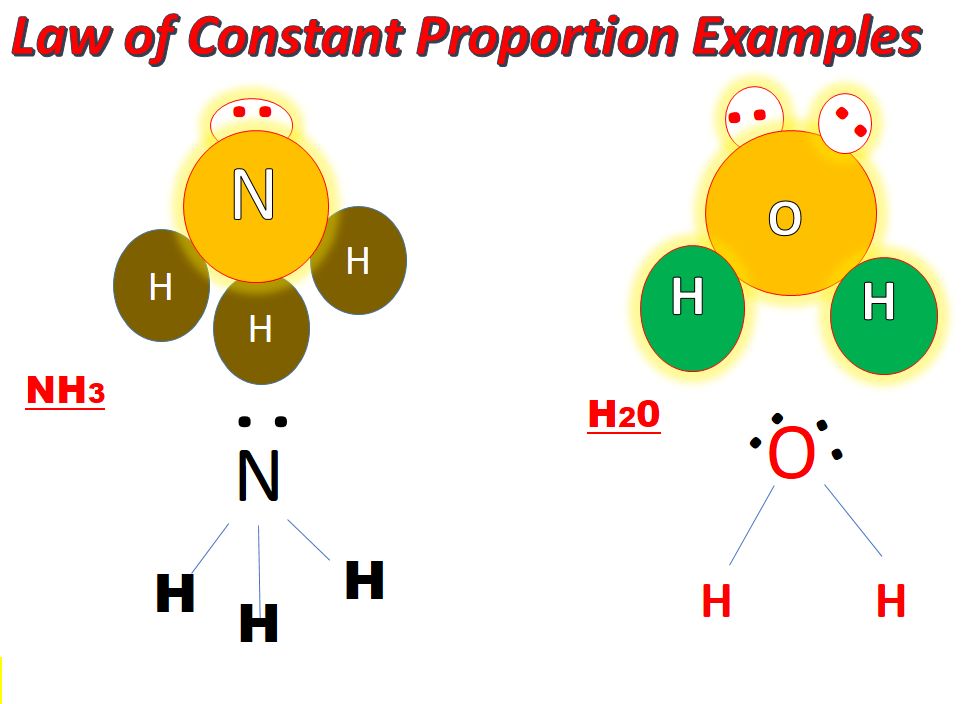
→ For example:
(i) 18 gm of H2O = 2 gm of hydrogen + 16 gm of oxygen
⇒ mass of hydrogen/mass of oxygen = 2/16 = 1/8
(ii) 36 gm of H2O = 4 gm of hydrogen + 32 gm of oxygen
⇒ mass of hydrogen/mass of oxygen = 4/32 = 1/8
(iii) 9 gm of H2O = 1 gm of hydrogen + 8 gm of oxygen
⇒ mass of hydrogen/mass of oxygen = 1/8
This verifies law of constant proportions as the ratio of mass of hydrogen to oxygen is always same.
2. IUPAC and atomic Symbols
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Dalton's Atomic Theory
→ According to Dalton’s atomic theory, all matter, whether an element, a compound or a mixture is
composed of small particles called atoms.
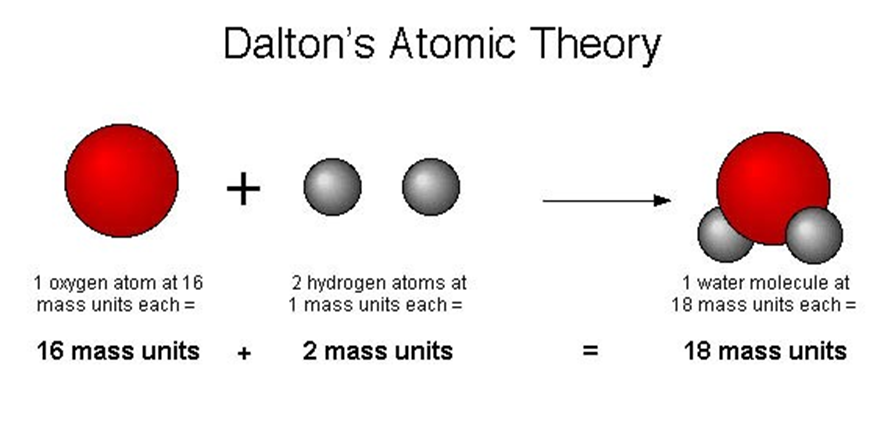
→ Six Postulates of Dalton's atomic theory:
(i) All matter is made of very tiny particles called atoms.
(ii) Atoms are indivisible particles, which cannot be created or destroyed in a chemical reaction.
(iii) Atoms of a given element are identical in mass and chemical properties. (Law of conservation of mass)
(iv) Atoms of different elements have different masses and chemical properties.
(v) Atoms combine in the ratio of small whole numbers to form compounds. (Law of constant proportion)
(vi) The relative number and kinds of atoms are constant in a given compound.
3. Compound
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Atoms
• Atoms are building blocks of all matter.
• According to modern atomic theory, an atom is the smallest particle of an element which taking
part in chemical reaction.
• Atoms are very small and which can’t be seen even through very powerful microscope.
• Atomic radius is measured in nanometres. Nanometres = 10-9 m.
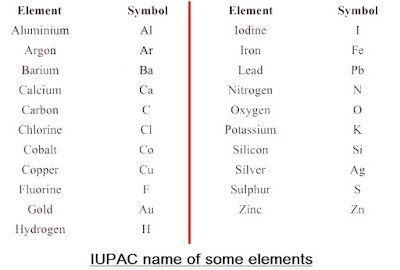
• Modern day symbols of Elements
→ Dalton was the first scientist to use the symbols for elements.
→ Berzilius suggested that the symbols of elements should be made from one or two letters of
name of the element.
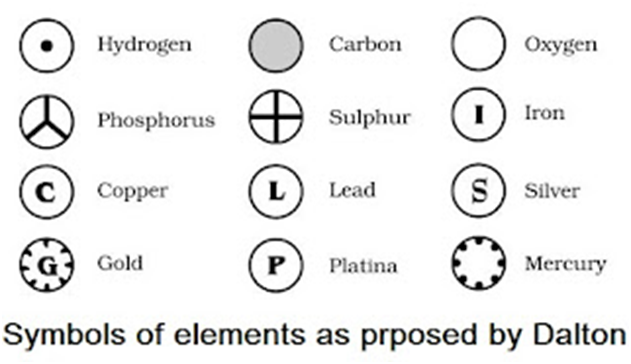
→ The name copper was taken from Cyprus, a place from where it was found for first time.
→ Now, IUPAC (International Union of Pure and Applied Chemistry) approves names of element
→ The first letter of a symbol is always written as a capital letter (uppercase) and the second letter.
as a small letter (lowercase). For example: hydrogen (H), aluminium (Al), cobalt (Co).
→ Some other symbols have been taken from the names of elements in Latin, German or Greek.For
example: Fe from its Latin name ferrum, sodium is Na from natrium, potassium is K from kalium.
• Atomic Mass
→ Dalton’s atomic theory proposed the idea of atomic mass which explained the law of constant
proportions so well.
→ The mass of an atom of an element is called its atomic mass.
→ In 1961, IUPAC have accepted ‘atomic mass unit’ (u) to express atomic and molecular mass of
elements and compounds.
→ The atomic mass unit is defined as the quantity of mass equal to 1/12 of mass of an atom of
carbon-12.
1 amu or u = 1/12 × Mass of an atom of C -12
1 u = 1.66 × 10-27 kg

• Atom existence
→ Atoms of most of the elements are very reactive and does not exist in free state.
→ Only the atoms of noble gases (such as He, Ne, Ar, Kr, Xe and Rn) are chemically unreactive and
can exist in the free state as single atom.
→ Atoms of all other elements combine together to form molecules or ions.
4. Chemical Formula
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
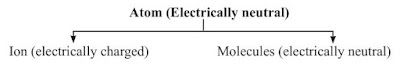
Molecules
→ A molecule is in general a group of two or more atoms that are chemically bonded together.
→ A molecule is the smallest particle of matter (except element) which is capable of an independent existence and show all properties of that substance.
→ Examples: ‘H2O’ is the smallest particle of water which shows all the properties of water.
→ A molecule may have atom of same or different elements, depending upon this, molecule can be categorized into two categories:
(i) Homoatomic molecules (containing atom of same element)
Examples: H2, O2, O3 , S8 , P4 etc.
(ii) Heteroatomic molecules or compounds (containing atoms of different elements)
Examples: H2O, CO2 , NaCl, CaCO3 etc.
5. Molecular Mass And Mole Concept
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
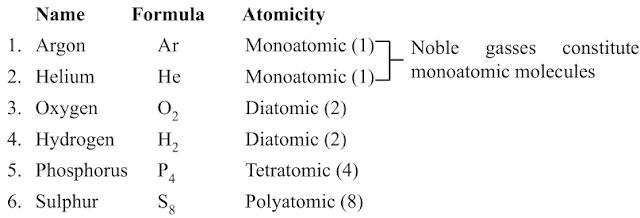
Atomicity
→ The number of atoms present in one molecule of an element is called its atomicity.
CHEMICAL FORMULAE
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Chemical Formulae
→ It is the symbolic representation of the composition of a compound.
• Characteristics of chemical formulae
→ The valencies or charges on ion must balance.
→ When a compound is formed of metal and non-metal, symbol of metal comes first. E.g., CaO
NaCl, CuO.
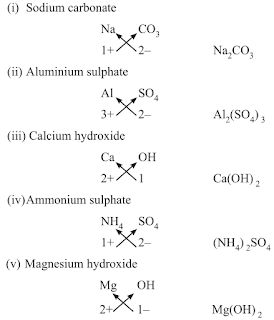
→ When polyatomic ions are used, the ions are enclosed in brackets before writing the numbe
show the ratio. E.g., Ca(OH)2, (NH4)2SO4
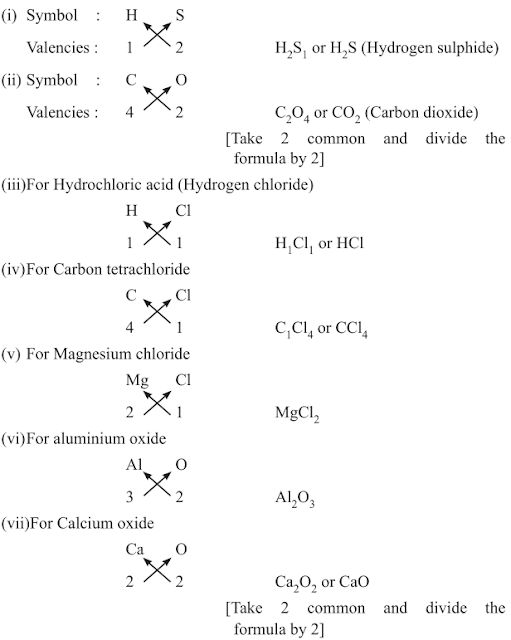
• Rules for writing chemical formulae
(i) We first write symbols of elements which form compound.
(ii) Below the symbol of each element, we should write their valency.
(iii) Now cross over the valencies of combining atoms.
(iv) With first atom, we write the valency of second atom (as a subscript).
(v) With second atom, we write valency of first atom (subscript).
MOLECULAR MASS
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Molecular Mass
→ It is the sum of atomic masses of all the atoms in a molecule of that substance.
Example: Molecular mass of H2O = 2×Atomic mass of Hydrogen + 1×Atomic mass of Oxygen
So, Molecular mass of H2O = 2×1 + 1×16 = 18 u
Formula Unit Mass
→ It is the sum of atomic mass of ions and atoms present in formula for a compound.
Example: In NaCl,
Na = 23 a.m.u.
Cl = 35.5 a.m.u.
So, Formula unit mass = 1×23
+ 1×35.5 = 58.5 u
IONS AND MOLE CONCEPT
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Ions
→ An ion may be defined as an atom or group of atoms having positive or negative charge.
→ Some positively charged ions : Na+ , K+ , Ca2+ , Al3+
→ Some negatively charged ions : Cl- (chloride ion), S2- (sulphide ion), OH-(hydroxide ion),
SO42- (sulphate ion)
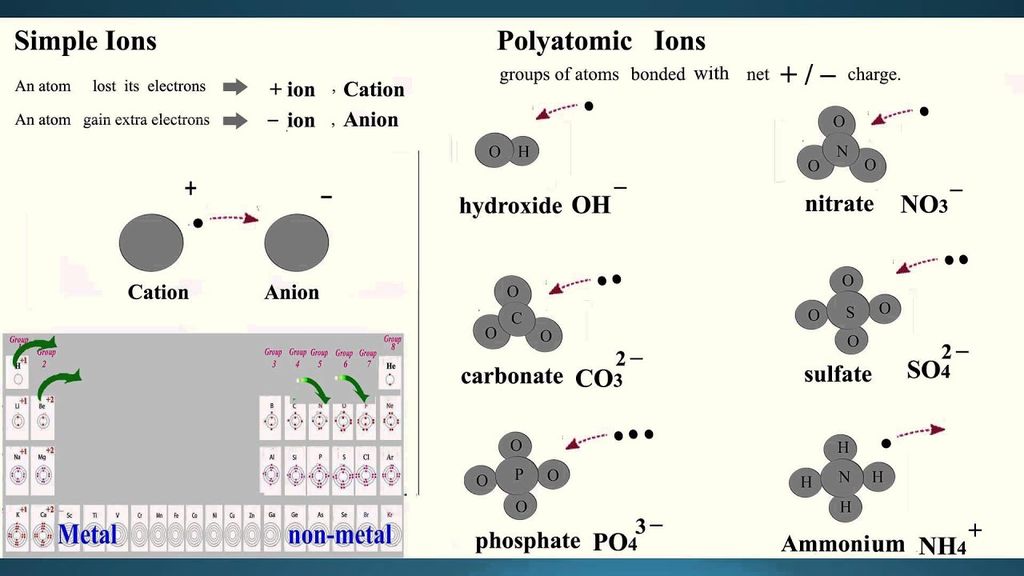
• We can classify ions in two types:
(i) Simple ions
Mg2+ (Magnesium ion)
Na+(Sodium ion)
Cl-(Chloride ion)
Al3+ (Aluminium ion)
(ii) Compound ions
NH4+ (Ammonium ion)
CO32- (Carbonate ion)
SO42- (Sulphate ion)
OH- (Hydroxide ion)
• Chemical Formulae of Ionic Compounds (Polyatomic)
Mole Concept
→ A group of 6.022×1023 particles (atoms, molecules or ions) of a substance is called a mole of
substance.
→ 1 mole of atoms = 6.022×1023 atoms
→ 1 mole of molecules = 6.022 × 1023 molecules
Example, 1 mole of oxygen = 6.022×1023 oxygen atoms
Note: 6.022×1023 is Avogadro Number (L).
→1 mole of atoms of an element has a mass equal to gram atomic mass of
the element.
Molar Mass
→ The molar mass of a substance is the mass of 1 mole of that substance.
→ It is equal to the 6.022×1023 atoms of that element/substance.
Examples:
(a) Atomic mass of hydrogen (H) is 1 u. Its molar mass is 1 g/mol.
(b) Atomic mass of nitrogen is 14 u. So, molar mass of nitrogen (N) is 14 g/mol.
(c) Molar mass of S8 = Mass of S×8 = 32×8 = 256 g/mol
(d) Molar mass of HCl = Mass of H + Mass of Cl = 1 = 35.5 = 36.5 g/mol
Important Formulae -
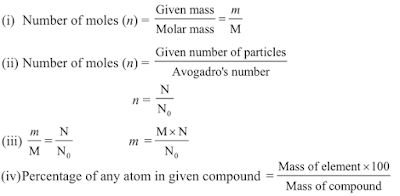
INTRODUCTION
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Introduction
→ John Dalton considered atom to be an indivisible entity, but his concept had to be discarded the end of nineteenth century,through experiments were able to find existence of charged (electrons and protons) and neutral particles (neutrons) in the atom. These particles are called the ‘Sub-atomic Particles’.
1. Introduction
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
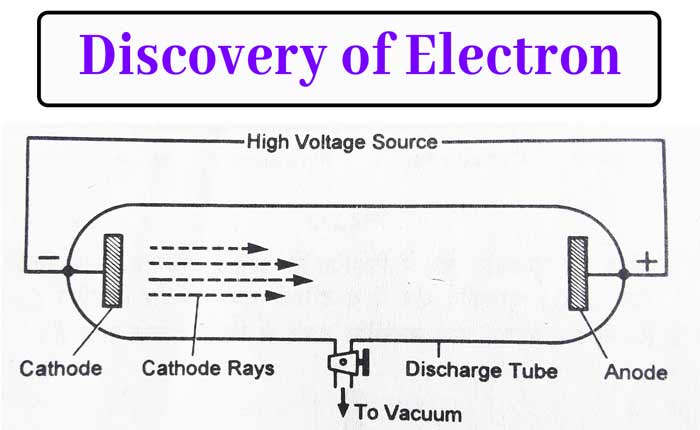
Discovery of Electrons – Cathode Rays (By J. J. Thomson)
→ Thomson explained presence of electrons by cathode rays experiment.
Facts about Electrons
→ Charge on electron = −1.6 × 10-19 C (C = Coloumb)
(As calculated by Robert E. Millikan)
→ Mass of electron = 9.1 × 10-31 kg
2. Discovery of Electrons and Protons
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
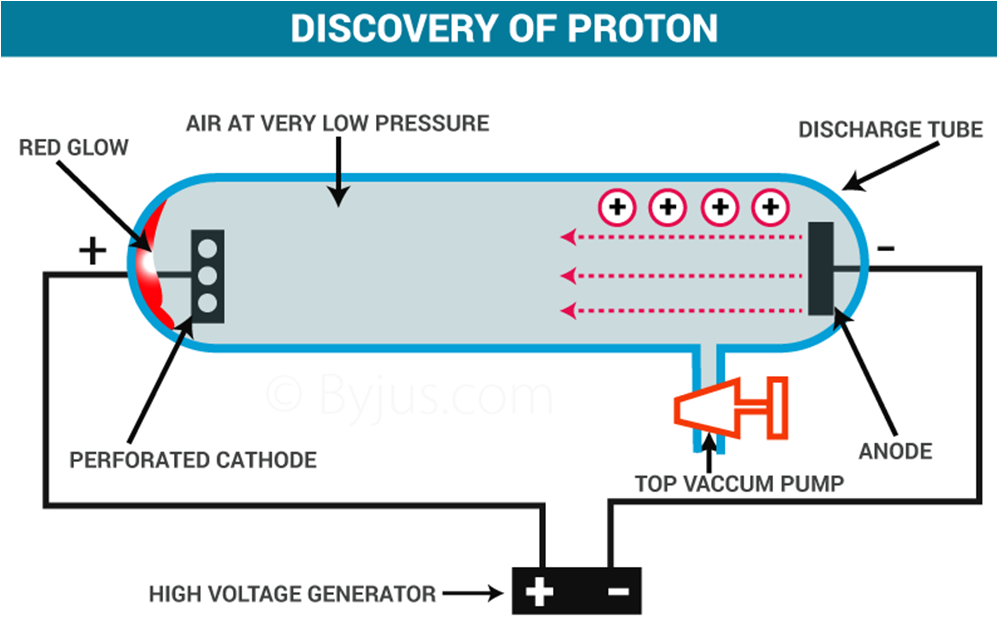
Discovery of Protons – Anode Rays/Canal Rays (By E. Goldstein)
→ E. Goldstein by his famous anode rays/canal rays experiment was able to detect presence of positively charged particles called protons in the atom.
Facts about Protons
→ Charge on proton = + 1.6 × 10-19 C
→ Mass of proton = 1.673 × 10 -24 gm
i.e., Mass of proton ≅ 1840 × Mass of electron
3. Discovery of Neutrons
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Discovery of Neutrons (By J. Chadwick)
→ J. Chadwick bombarded lighter elements (like lithium, boron etc.) with α-particles and observes
emission of new particles having zero charge but having mass equal to that of proton.
→ These particles were called ‘Neutron’ i.e., neutral particle of the atom.
→ Neutron are absent in Protium isotope of hydrogen atom.(1H1)
→ Since, mass of electrons are negligible as compared to that of proton and neutrons hence, sum
of masses of protons and neutrons in an atom will compose its atomic mass.
4. Some Important Defintion
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Atomic Models
→ From the knowledge of existence of subatomic particles like electron, proton and neutron in
atom, various atomic models were proposed by different scientists.
• Some of the atomic models:
(i) Thomson’s Model of Atom
(ii) Rutherford’s Model of Atom
(iii) Bohr’s Model of Atom
→ The most trusted and scientifically established model of atom which is adopted these days
‘Quantum Mechanical Model of Atom’. It will be dealt in higher classes.
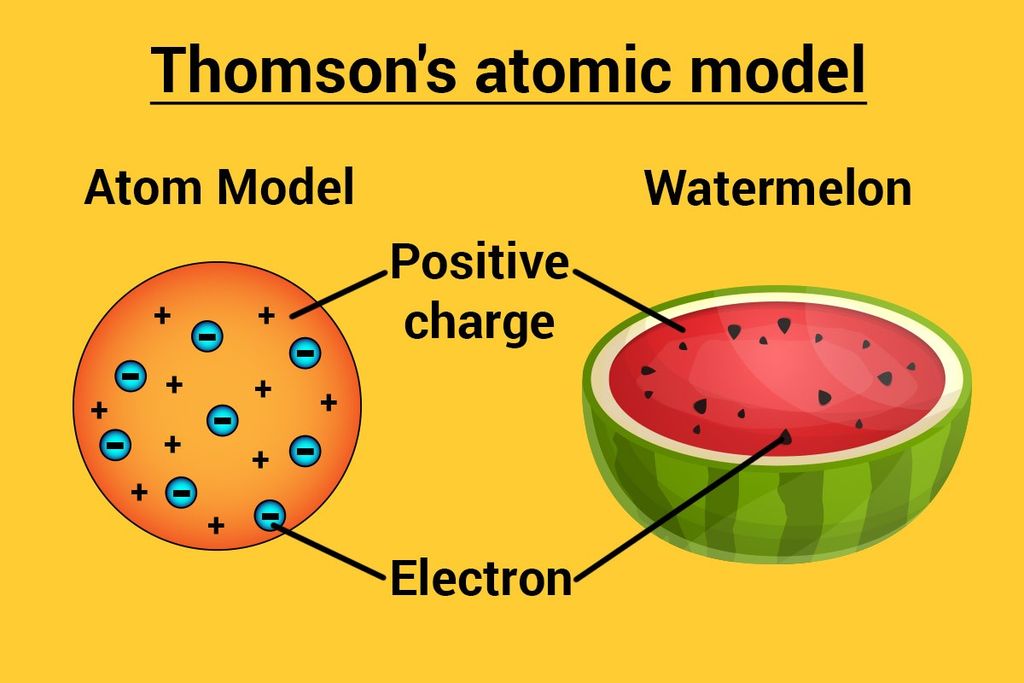
Thomson’s Atomic Model
→ This model is often called the ‘Water Melon Model’.
→ In this model, Thomson predicted the presence of electrons inside positive sphere (made up
protons), just same as seeds of watermelon are embedded in red edible part of watermelon.
→ Although this model explained neutrality of atom but couldn’t able to explain other scientific
experiments conducted on atom. Hence it was discarded.
5. Atomic Number And Mass Number
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Rutherford’s Atomic Model
→ In his famous ‘α-ray Scattering Experiment’, Rutherford bombarded α-ray (Helium nucleus 2 H
upon thin gold foil.
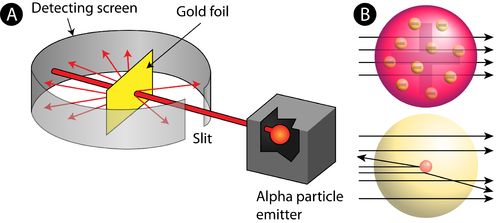
• Observations made by Rutherford in his experiment:
(i) Most of α-particles passed through gold foil undeflected.
(ii) Some of the α-particles deflected by foil by small angles.
(iii) One out of every 12000 particles appeared to rebound.
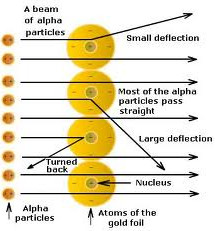
• Conclusions made by Rutherford:
(i) Atom consists of predominantly empty space as most of α-particles passed through gold foil undeflected.
(ii) Atom contains centrally placed positively charged nucleus (carrying positively charged particles), because few α-particles suffered deflected and very few i.e., one in 12000 bounced back.
(iii) Since a minute fraction of α-particles suffered deflections and very few bounced back, this
to conclusion that most of the space an atom is empty and the space occupied by nucleus is negligible compared to this empty space.
→ Size of nucleus was about 10-5 times that of size of atom.
(iv) Whole of the atomic mass concentrated in the nucleus.
• Features of Rutherford proposed model of atom:
(i) There is positively placed nucleus in an atom. Nearly all the mass resides in nucleus (Proton + Neutron).
(ii) Electrons revolves round the nucleus in well defined orbits.
(iii) Size of nucleus is very small compared to the size of atom.
• Drawbacks of Rutherford’s Model (Unstability of Atom)
→ According to Rutherford, electrons revolve round the nucleus in well-defined orbits, but elec
being charged particles will lose their energy and finally will fall into the nucleus.
→ This will make atom highly unstable.
→ This was the major drawback of Rutherford which was unexplained by him.
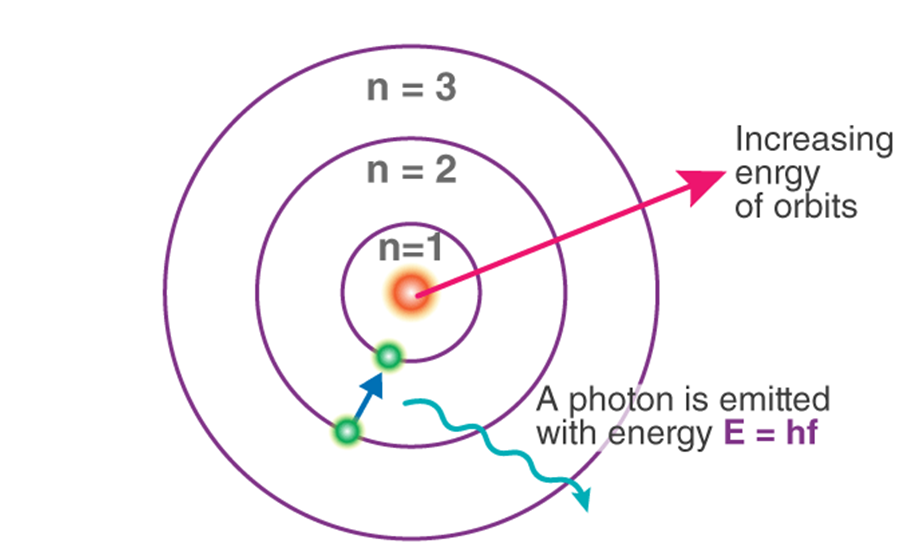
→ To overcome drawbacks of Rutherford’s Model, Neil Bohr in 1912 proposed modified model of
structure of atom.
Assumption made by Neil Bohr
→ Only certain special orbits known as discrete orbits of electrons are allowed inside the atom
→ While revolving in discrete orbits, the electrons do not radiate energy.
→ Energy is emitted or absorbed by an atom only when an electron moves from one orbit to another.
6. Isotopes
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Atomic Number
→ The total number of proton lying in the nucleus of any atom is called the atomic number.
→ An atomic number is the identity of an atom, changing atomic number means changing the atom.
→ Atomic number is denoted by ‘Z’.
→ Atomic number = no. of protons or a neutral atom, no. of protons and electrons are equal.
Mass Number
→ It is the sum of total number of protons and no. of neutrons lying in the nucleus of an atom.
→ It is denoted by ‘A’.
→ Mass number = no. of protons + no. of neutrons
→ Representation of an atom:
zXA
(X= symbol of an element)
Example: Calculate number of protons, electrons and neutrons for 17Cl35 or 17Cl37
Sol. Since Cl is neutral,
No. of electrons = no. of protons = 17
Mass no. of Cl = 35
No. of neutrons = 35 - 17 =18
Distribution Of Electrons In Various Shells
→ The distribution of electrons in various shells is done in accordance to ‘Bohr-Bury Scheme’.
Bohr-Bury Scheme
(i) The filling of electrons in an atom is done in accordance to ‘2n2’, where ‘n’ is the number of shell and ‘2n2 ’ represents the total number of electrons that can be accommodated in that particular shell.
→ Maximum number of electrons that can be filled in particular shell.
If n = 1, i.e., K = shell, 2n2= 2×1 2 = 2 electrons
If n = 2, i.e., L = shell, 2n2 = 2×2 2 = 8 electrons
If n = 3, i.e., M = shell, 2n2 = 2×3 2 = 18 electrons
If n = 4, i.e., N = shell, 2n2 = 2×4 2 = 32 electrons
(ii) The outermost shell can’t hold more than 8 electrons, while second last shell can’t have more than 18 electrons, even though they may have capacity to hold more electrons.
Example: ‘Ca 20 ’, the electron distribution will be :
Ca 20 = 2(K), 8(L), 8(M), 2(N)
→ But Ca 20 = 2, 8, 10 is wrong although ‘M’ shell can contain upto 18 electrons.
(iii) The outermost shell can’t hold more than 2 electrons and the penultimate shell can’t hold more than 8 electrons unless the preceding inner shell (antepenultimate shell) is filled completely obey ‘2n2 ’ rule.
(i) K(19) = 2, 8, 8, 1
(ii) Al (13) = 2, 8, 3
(iii) F (9) = 2, 7
(iv) Ne (10) = 2, 8
(v) Na (11) = 2, 8, 1
Valence Shell and Valence Electrons
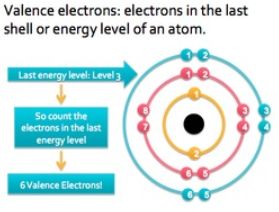
→ From Bohr-Bury sequence, we know that maximum number of electrons which can be accommodated in outermost shell is 8.
→ Every element has an urge to have 8 electrons in its outermost shell, in achieving 8 electrons atom can either gain electrons or loose electrons.
→ The number of electrons lost or gained by an element in achieving 8 electrons in its outermost shell will be called its Valency.
→ For elements like H, He, Li, Be and B, these elements lose their outermost electron to achieve 2 electrons in their outermost shell.
→ Isotopes are atoms of same elements having same atomic number and different mass number.
Example: Chlorine has two isotopes of mass numbers 35 and 37 respectively.
17Cl35 , 17Cl37
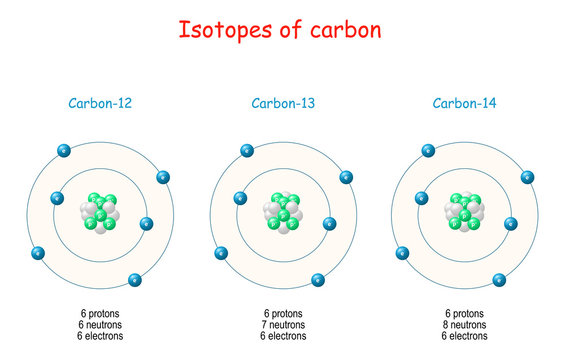
Uses of isotopes
(i) Uranium isotope is used as fuel in nuclear rector.
(ii) Isotope of cobalt is useful in treatment of cancer.
(iii) An isotope of iodine is used in the treatment of goitre.
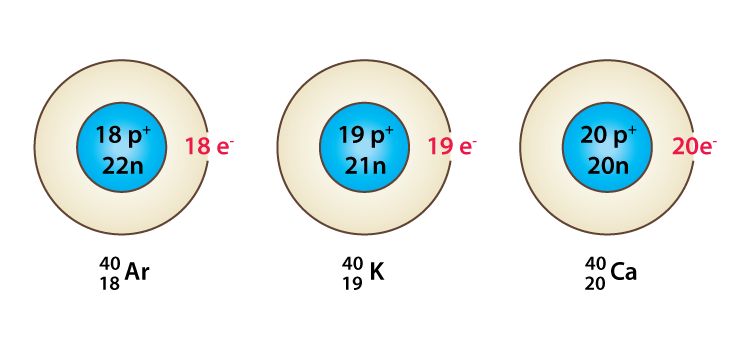
Isobars
→ Isobars are the atoms of those elements which have the same mass number but different atomic
numbers are called isobars.
→ 20Ca40 and 18Ar40 have same mass number and different atomic number. 11Na24 and 12Mg24
another examples.

 EduMple Learning
EduMple Learning
 ACERISE INDIA
ACERISE INDIA
