- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Types of pure substances
The pure substance is divided in two types on the basis of their chemical composition:
(i) Elements
(ii) Compounds
(i) Elements
• According to Antoine Laurent Lavoisier, element is a basic form of matter that cannot be broken
down into simpler substances by chemical reactions.
• It is divided in three types which are metals, non-metals and metalloids.
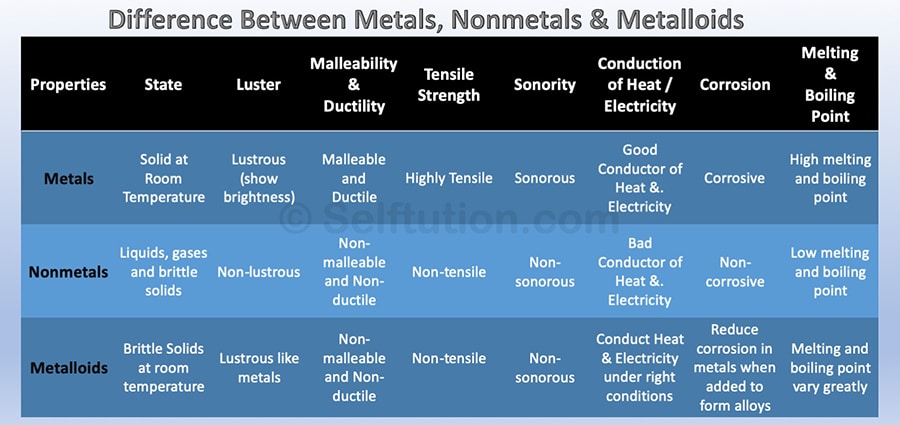
Properties of Metals
(i) They have a lustre (shine).
(ii) They have silvery-grey or golden-yellow colour.
(iii) They conduct heat and electricity.
(iv) They are ductile (can be drawn into wires).
(v) They are malleable (can be hammered into thin sheets).
(vi) They are sonorous (make a ringing sound when hit).
• Examples of metals are gold, silver, copper, iron, sodium, potassium etc.
• Mercury is the only metal that is liquid at room temperature.
Properties of non-metals
(i) They display a variety of colours.
(ii) They are poor conductors of heat and electricity.
(iii) They are not lustrous, sonorous or malleable.
• Examples of non-metals are hydrogen, oxygen, iodine, carbon (coal, coke), bromine, chlorine
Metalloids: Elements having intermediate properties between those of metals and non-metal
called metalloids. Examples are boron, silicon, germanium etc.
(ii) Compounds
A compound is a substance composed of two or more elements, chemically combined with oneanother in a fixed proportion.
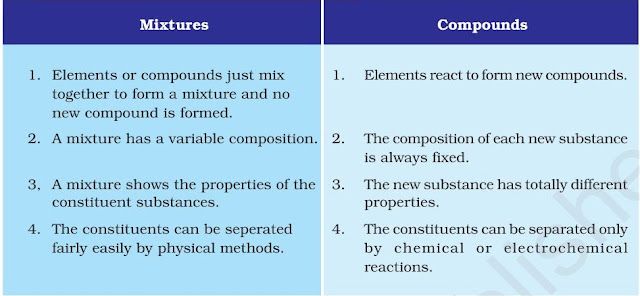
Difference between mixtures and compounds

 Science Made Easy
Science Made Easy
