1. Introduction
- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
Chapter-4
Structure of the Atom
Structure of the Atom Introduction
As you all know, we come across different substances around us that constitute matter. This matter is made up of small particles called atoms. Let us know more about atom, its structure and its constituents. Matter: is anything that occupies space and has mass. It consists of tiny particles which were called ‘parmanu’ but later on this name was replaced by ‘atom’.
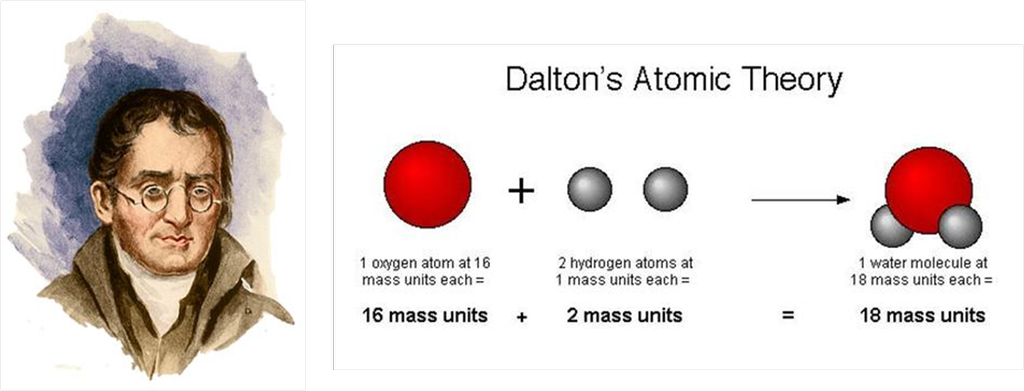
Dalton’s Atomic Theory
The scientist, Dalton was the first one to actually formulate all information of an atom in a theoretical form as Dalton’s Atomic Theory. He was born in 1766 and died in 1844. His theory was published in 1808. His full name was John Dalton
According to this theory, the following observations were made
1. All matter is made up of small particles called atoms.
2. Atom is invisible.
3. Atom is indivisible.
4. Atoms of an element are alike in all aspects, that is, if we talk about sodium then all the atoms of sodium will be the same in all aspects.
5. Atoms of different elements are different, that is, if we talk about sodium and potassium, then, the atoms of both are going to be different but same among themselves.
6. Atoms of different elements combine in a fixed simple whole number ratio to form compounds.
7. Atoms can neither be created nor destroyed, that is, the origin of atoms is not known.
Drawbacks of Dalton’s Atomic Theory
There were certain limitations as observed by other scientists. They observed the following
1. According to Dalton, an atom was indivisible but later on, it was proved that atom can be subdivided into sub atomic particles called electrons, protons & neutrons.
2. Atoms of the same element can somehow differ from each other. This was proved due to the existence of isotopes in nature.
3. Similarly, atoms of different elements can be the same. This came into notice due to the existence of isobars in nature.
4. According to it, whenever the compound is formed, it is formed as a result of the combination of atoms in a fixed simple ratio. But it has been seen that the ratio might not always be simple. For example, in sucrose that is C12H22O11, the ratio is not a simple ratio.
So, these drawbacks led to failure of Dalton’s theory of an atom.
2. Discovery of Electrons and Protons
- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
Discovery of Electrons and Protons
Discovery of electrons
It was discovered by J.J. Thomson in the year 1897. He took Crooke’s tube and arranged the apparatus as shown in figure:
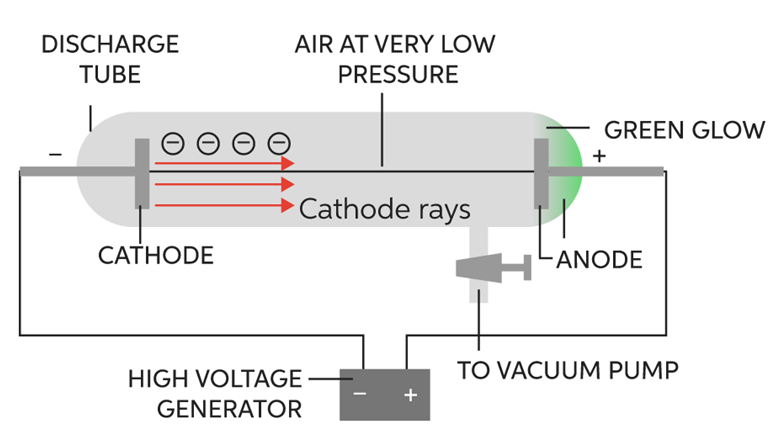
The following observations were made
1. When he passed electric current (at high voltage approximately at 10,000volts) through a gas at a pressure of 1 atm, then nothing happened as no changes were seen.
2. When he reduced the pressure to 10-2 atm, the whole tube started glowing with green colour.
3. He further reduced the pressure to 10-4 atm, the whole tube stopped glowing, but a faint green colour was still seen at the anode end.
4. To confirm, a fluorescent screen was placed at the back of the anode and anode was made perforated. When current was passed through it (in the same physical conditions), the Zinc Sulphide screen started glowing which confirmed the following fact.
The following was concluded
It proved that at these conditions, some rays were emitted through cathode and were travelling towards anode called cathode rays consisting of negatively charged particles. These particles were later called electrons. This is how the electron was discovered.
Discovery of Protons
It was discovered by E.Goldstein in the year 1920. It was actually discovered while carrying out an experiment to produce cathode rays. The apparatus used was the same:
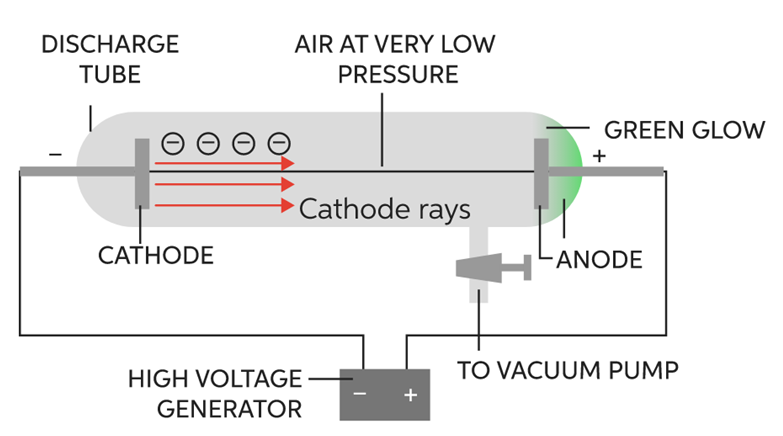
The following observations were made
It was seen that when cathode rays were produced (at high voltage and low pressure), they travelled through the gas in the discharge tube. While doing so, they ionized the gas. That is, they took electrons of gas along, leaving behind positively charged particles of the gas. These particles formed canal rays and started moving towards the cathode. These particles are called canal rays as they are not produced by anode. As these particles possess positive charge so they were named as protons.
Now after knowing about the atom, different attempts were made to know about its structure.
Thomson’s model of an atom (plum pudding model)
His full name was J.J. Thomson and he was the one who made the first attempt to explain the structure of atoms.
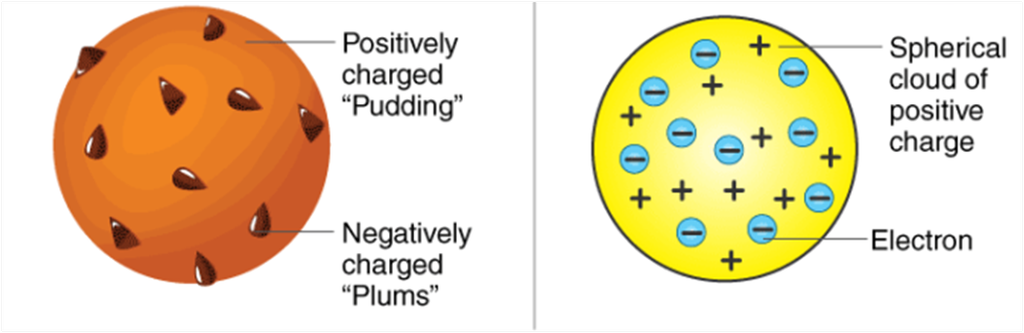
According to him, an atom is a positively charged sphere in which negative charges are present at certain places like plums in a pudding or cherries in an ice-cream. But this model was rejected as he could not explain the major point seen in his model.
Drawback of Thomson’s atomic model were as follows
He could not explain the distribution of charges and stability of an atom. As we all know that opposite charges attract each other, so, how come it is possible that few negative charges remain scattered in this big positive space. They would have been neutralized. This could not be explained by J.J. Thomson that lead to failure of his attempt.
Rutherford’s scattering experiment
In order to understand the structure of an atom, Rutherford performed the scattering experiment. For this, he took a gold foil and passed alpha rays through it. Gold foil actually consists of many gold atoms. So, at an individual level, we are considering the observations through an atom. Alpha rays are actually positively charged rays consisting of Helium nucleus (He).
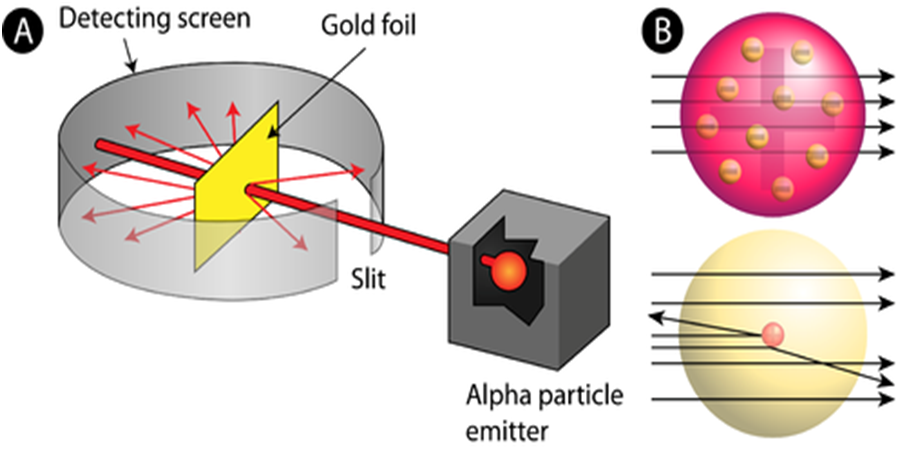
These rays, when passed through it suffered reflections at different angles and to note that a movable screen made of fluorescent material was placed around it. When the reflected rays strike that screen it causes scintillation. When he passed these rays through gold foil various observations were seen.
Observations were as follows
- Most of the rays passed straight.
- Some rays were deflected through small & large angles.
- Some rays rebound back.
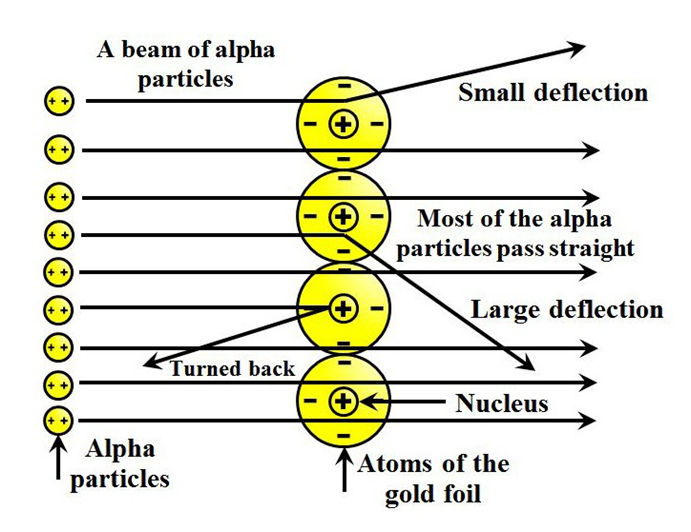
The following conclusions were made
- Most of the space in an atom is empty.
- There is something in the centre of an atom called nucleus.
- Nucleus is positively charged.
So, According To Rutherford, the structure of an atom is similar to that of the solar system.
He said:
- Atom is electrically neutral
- Nucleus is in centre in which protons are present.
- Outside nucleus electrons revolve like planets revolve around the Sun.
In this model also few limitations were seen and some questions were left unanswered, which led to its failure.
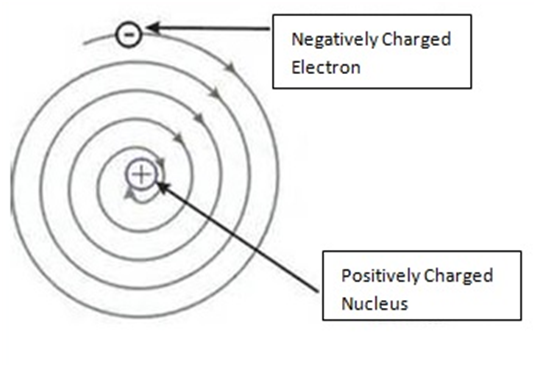
The drawback of Rutherford’s experiment were as follows –
He failed to explain the stability of an atom”.
According To electromagnetic theory: Any charged particle, when revolves in a circular path, continuously emits energy and shortens its path. As we know, an electron is also a charged particle revolving in a circular path so it should also emit energy, shorten its path and should finally falls into the nucleus and for doing so, it needs time lesser than a fraction of a second. But this doesn’t happen.
3. Discovery of Neutrons
- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
Discovery of Neutrons
Discovery of neutron
It was discovered by Chadwick in the year 1932. It was actually discovered while considering the mass of atomic particles. It was seen that the whole mass of an atom is due to the nucleus and as far we know, the nucleus is positively charged. That means it has only protons in it. But we also know that the mass of an atom is never equal to the number of protons. This shows that the nucleus contains some other particles also that contribute towards mass only and not towards charge. Therefore, the particles were called neutrons (as they possess no charge).
Bohr’s theory
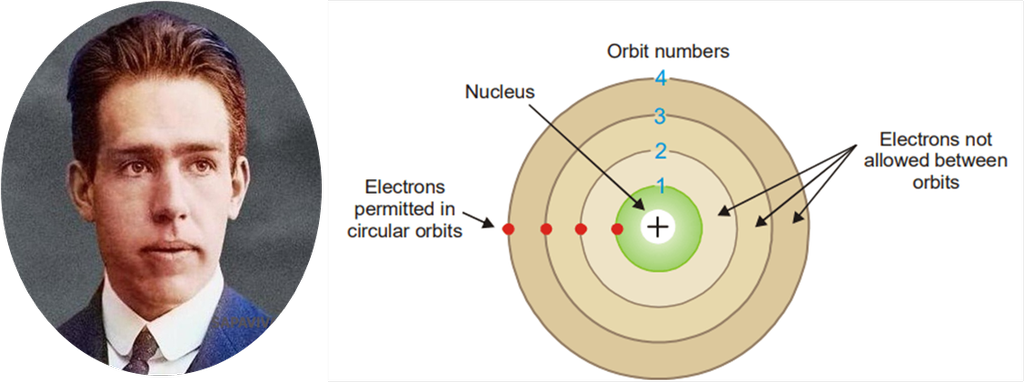
To overcome the limitations of Rutherford model, the new concept and picture of atom was given by Neil Bohr which made a great contribution in knowing the structure of an atom. According to it:
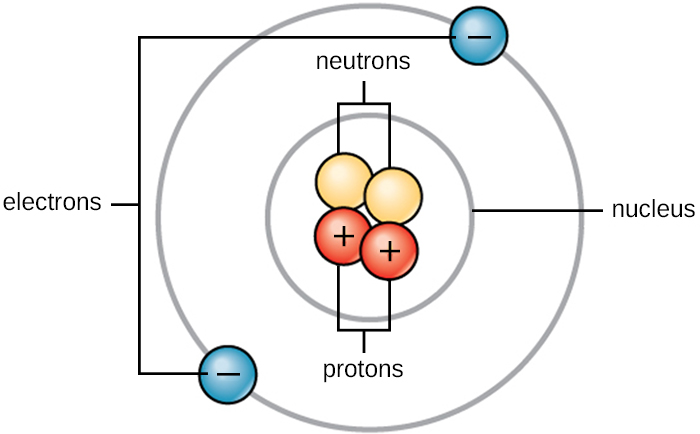
- Atom is electrically neutral i.e. number of Protons = number of Electrons.
- In the centre of an atom, nucleus is present which is positively charged.
- In the nucleus, protons and neutrons are present.
Protons possess = positive
Neutron possess = no charge
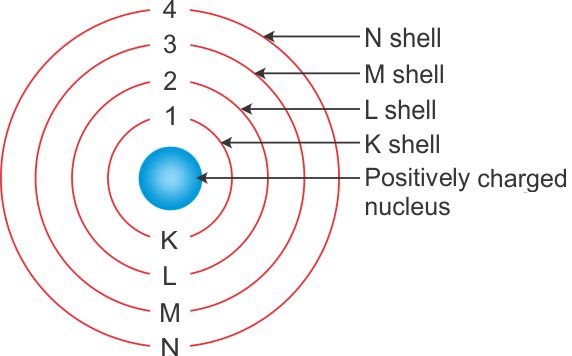
- Outside the nucleus, shells or energy levels designated as K,L,M,N and so on are present.
- In shells, electrons revolve.
- Electrons are negatively charged.
- Each shell has a fixed amount of energy. So, as long as an electron remains in the same shell, it never loses or gains energy.
- Number of electrons in each shell is determined by Bohr Bury rule i.e. 2n2.
4. Some Important Defintion
- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
Some Important Defintion
Atomic number (z): Is defined as “Number of protons in atom”.
Mass number (A): Is defined as “sum of protons and neutrons present in the nucleus of an atom.”
Representation of an element
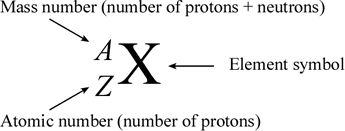
Where Z = atomic number (Sub script)
Where A= mass number (Superscript)
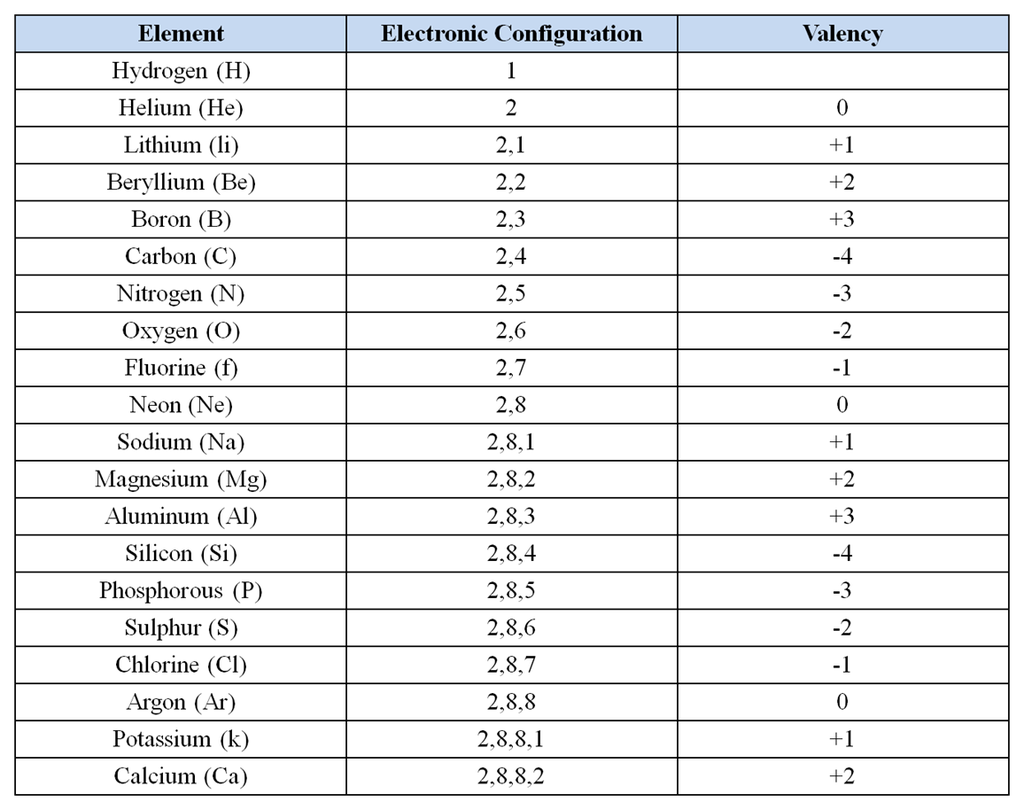
Electronic configuration It is defined as “Arrangement of electrons in different shells.” The arrangement is according to Bohr bury rule that is 2n².
Valence shell It is the last shell of an atom. For example, in the case of Sodium, the electronic configuration is 2,8, 1. In this, the shells involved are K,L,M. Therefore, the last shell is M (valence shell).
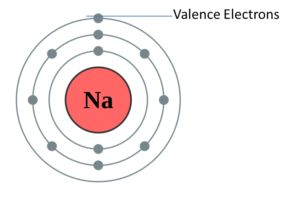
Valence electrons The electrons present in the valence shell of an atom, for example, in the case of Sodium, the electronic configuration is 2,8, 1. In this, the shells involved are K,L,M. The electrons present in valence shell are valence electrons that are 1.
Valency It is the combining capacity of an atom. It depends upon the number of valence shell electrons that is electrons in the last shell of an atom (that can be seen while writing electronic configuration). We have two types of valency that is:
- Electro-valency
- Covalence
- Electro-valency: It is the valency that is attained by losing and gaining of electrons. For example, in the case of Sodium, the atomic number is 11 and electron distribution is 2,8,1. So, in order to attain stability, it can either lose one electron or gain 7 electrons. Out of the two oprions, losing 1 electron is easier, therefore, it loses one electron and therefore, its valency is +1.
- Covalence: It is the valency that is attained by sharing of electrons. For example, in the case of carbon, it can attain stability by sharing 4 electrons. If we look at its distribution, it needs four more electrons, so it completes its octet by sharing four electrons. Therefore, its valency is -4.
Isotopes
They are those elements which have the same atomic number but different mass numbers.
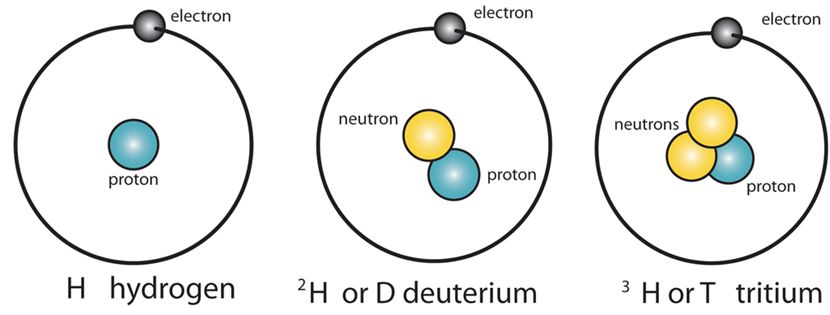
Properties of isotopes are as follows
- The number of protons is the same.
- The symbol is the same.
- Number of valence electrons and valency is same.
- They differ only in number of neutrons.
Applications of isotopes
- U-235 is used as a nuclear fuel.
- Co-60 used in the treatment of cancer.
- I-128 used in the treatment of goitre.
- C-14 is used in carbon dating.
For example:
- In the case of Hydrogen, the isotopes are – H1 H2 H3 . (But atomic number for all is 1).
- In case of oxygen, the isotopes are – O16 O17 O18 . (But atomic number for all oxygen is 8).
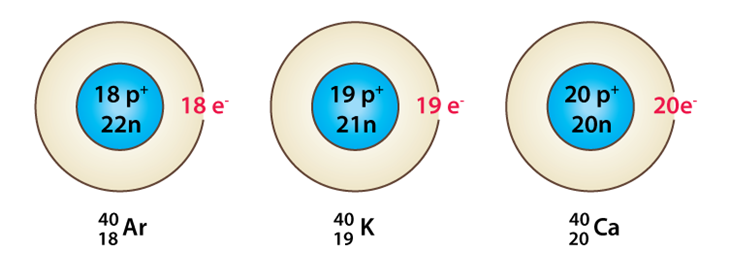
Isobars
They are those elements which have the same mass number but different atomic number. For example: sodium and magnesium are isobars as they have different atomic numbers but same mass number that is 24.
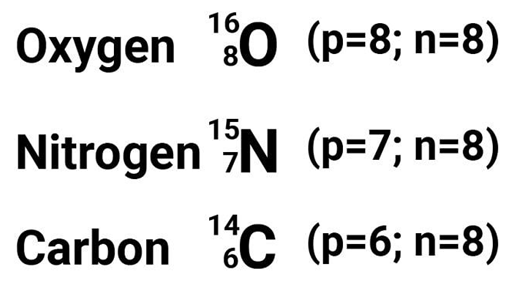
Isotones
They are those elements which have the same number of neutrons. For example: carbon, nitrogen and oxygen all have 8 neutrons.
The atomic numbers of various elements along with electronic configuration and valency is given below
Atomic numbers from 1 to 20 are as follows

 Science Made Easy
Science Made Easy
 ACERISE INDIA
ACERISE INDIA
