- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Molecules
→ A molecule is in general a group of two or more atoms that are chemically bonded together.
→ A molecule is the smallest particle of matter (except element) which is capable of an independent existence and show all properties of that substance.
→ Examples: ‘H2O’ is the smallest particle of water which shows all the properties of water.
→ A molecule may have atom of same or different elements, depending upon this, molecule can be categorized into two categories:
(i) Homoatomic molecules (containing atom of same element)
Examples: H2, O2, O3 , S8 , P4 etc.
(ii) Heteroatomic molecules or compounds (containing atoms of different elements)
Examples: H2O, CO2 , NaCl, CaCO3 etc.
Chemical Formulae
Rules:
(i) The valencies or charges on the ion must balance.
(ii) Metal and non-metal compound should show the name or symbol of the
metal first.
e.g., Na+ Cl– → NaCl
(ii) If a compound consists of polyatomic ions. The ion is enclosed in a bracket before writing the number to indicate the ratio.
e.g., [SO4]2- → polyatomic radical
H1+ SO42- → H2SO4
Molecular Mass
It is the sum of the atomic masses of all the atoms in a molecule of the substance. It is expressed in atomic mass unit (u).
Formula Unit Mass
It is the sum of the atomic masses of all atoms in a formula unit of a compound. The constituent particles are ions.
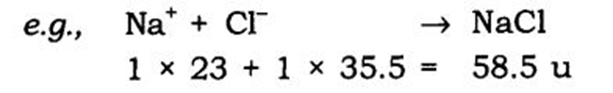
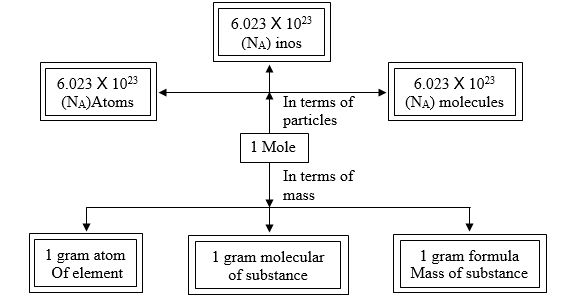
Mole Concept
Definition of mole: It is defined as one mole of any species (atoms, molecules, ions or particles) is that quantity in number having a mass equal to its atomic or molecular mass in grams.
1 mole = 6.022 x 1023 in number
Molar mass = mass of 1 mole → is always expressed in grams and is also known as gram atomic mass.
l u of hydrogen has → 1 atom of hydrogen and 1g of hydrogen has → 1 mole of hydrogen
= 6.022 x 1023 atoms of hydrogen.
- Books Name
- Yash Tyagi Coaching Science Book
- Publication
- ACERISE INDIA
- Course
- CBSE Class 9
- Subject
- Science
Chemical Formula
Chemical formula
Chemical Formula is the symbolic representation of compound. For example, the compound ammonium nitrate is written as
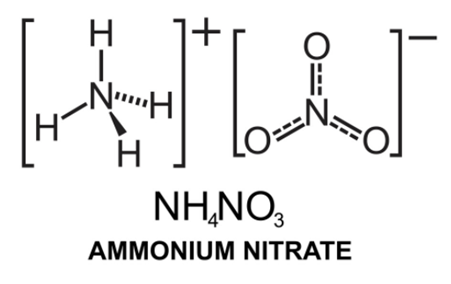
4NO3) - Structure, Preparation, Physical and Chemical Properties, Uses with FAQs of Ammonium Nitrate" style="width:286px; height:179px" data-cke-upload-id="0" data-widget="uploadimage">
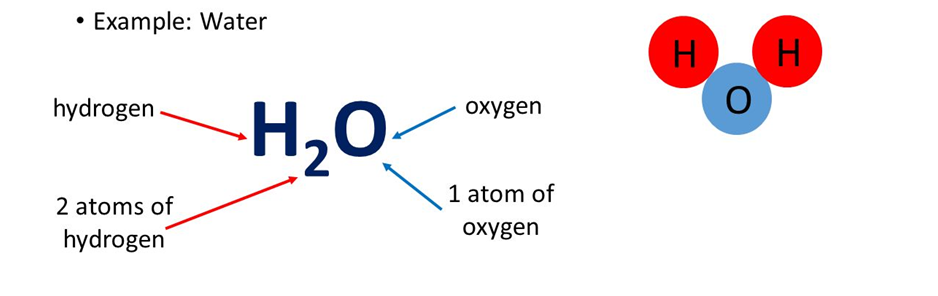
If we say that water is represented as H2O, we get the following information from it
1. H2O represents water.
2. H2O represents one molecule of water.
3. It represents that 1molecule of H2O contain 2Hydrogen and 1 Oxygen atoms.
4. It represents 18g of water.
5. It represents that 1 molar mass of water has 6.022 x 10 23 molecules.
Mass percentage
Mass Percentage is the ratio of mass of a substance whose percentage is to be calculated to the total mass of the substance multiplied by 100.
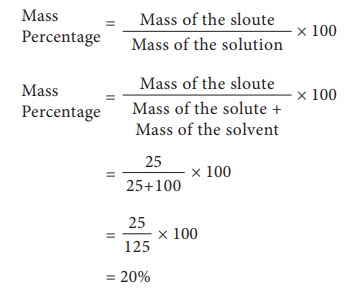
Example 1: Find the mass percentage of 6 g sodium hydroxide dissolved in 50 g of water. (Note: since the density of water is nearly 1, this type of question often gives the volume of water in milliliters.)
First find the total mass of the solution:
total mass = 6 g sodium hydroxide + 50 g water
total mass = 56 g
Now, you can find the mass percentage of the sodium hydroxide using the formula:
mass percent = (grams of solute / grams of solution) x 100
mass percent = (6 g NaOH / 56 g solution) x 100
mass percent = (0.1074) x 100
answer = 10.74% NaOH
Example 2: Find the masses of sodium chloride and water required to obtain 175 g of a 15% solution.
This problem is a bit different because it gives you the mass percentage and asks you to then find how much solute and solvent are needed to yield a total mass of 175 grams. Start with the usual equation and fill in the given information:
mass percent = (grams solute / grams solution) x 100
15% = (x grams sodium chloride / 175 g total) x 100
Solving for x will give you the amount of NaCl:
x = 15 x 175 / 100
x = 26.25 grams NaCl
So, now you know how much salt is needed. The solution consists of the sum of the amount of salt and water. Simply subtract the mass of salt from the solution to obtain the mass of water that is required:
mass of water = total mass - mass of salt
mass of water = 175 g - 26.25 g
mass of water = 147.75 g
Mole Concept
Mole Concept is equal to 6.023 x 1023 entities present in a substance. It is also called Avagadro’s number. Mole = given mass of substance to its atomic or molecular mass.
Example 1: Calculate the number of moles for the following:
(i) 52 g of He (finding mole from mass)
(ii) 12.044 × 1023 number of He atoms (finding mole from number of particles).
Solution:
No. of moles = n
Given mass = m
Molar mass = M
Given number of Particles = N
Avogadro number of Paticles = N0
(i) Atomic mass of He = 4u
Molar mass of He = 4g
Thus, the number of moles = given mass/molar mass
n = m/M = 52/4 = 13
(ii) we know,
1 mole = 6.022 × 1023
The number of moles
= given number of particles/Avogadro number
n=N/N0= 12.044 × 1023/6.022 × 1023= 2
Example 2: Calculate the mass of the following:
(i) 0.5 mole of N of molecule)
(ii) 0.5 mole of N atoms (mass from mole of atom)
(iii) 3.011 × 1023 number of N atoms (mass from number)
(iv) 6.022 × 10 23 number of N2 molecules (mass from number)
Solution:
(i) mass = molar mass × number of moles
m = M x n = 28 0.5 = 14 g
(ii) mass = molar mass × number of moles
⇒ m = M × n = 14 × 0.5 = 7 g
(iii) The number of moles, n
= given number of particles/Avogadro number =N/N0
= 3.011 × 1023/6.022 × 1023
m=M x n =14 x 3.011 × 1023/6.022 × 1023
=14×0.5 =7 g
(iv) n =N/N0
m= M x N/N0= 28 x 6.022 × 1023/6.022 × 1023
=28×1=28 g

 Science Made Easy
Science Made Easy
 ACERISE INDIA
ACERISE INDIA
