INTRODUCTION
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Introduction
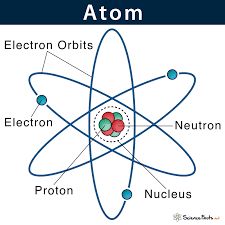
→ John Dalton considered atom to be an indivisible entity, but his concept had to be discarded the end of nineteenth century,through experiments were able to find existence of charged (electrons and protons) and neutral particles (neutrons) in the atom. These particles are called the ‘Sub-atomic Particles’.
1. Introduction
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
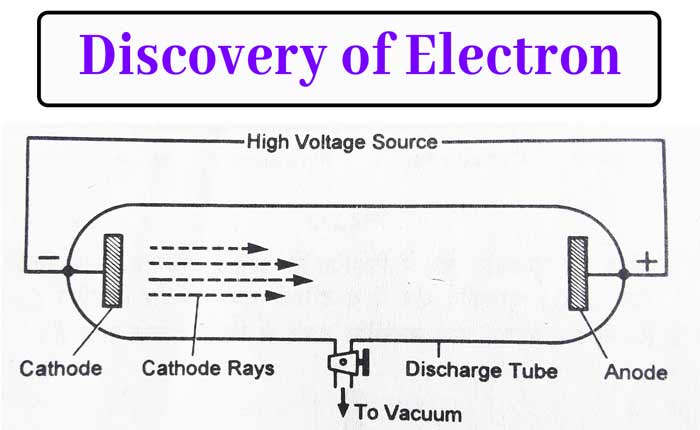
Discovery of Electrons – Cathode Rays (By J. J. Thomson)
→ Thomson explained presence of electrons by cathode rays experiment.
Facts about Electrons
→ Charge on electron = −1.6 × 10-19 C (C = Coloumb)
(As calculated by Robert E. Millikan)
→ Mass of electron = 9.1 × 10-31 kg
2. Discovery of Electrons and Protons
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
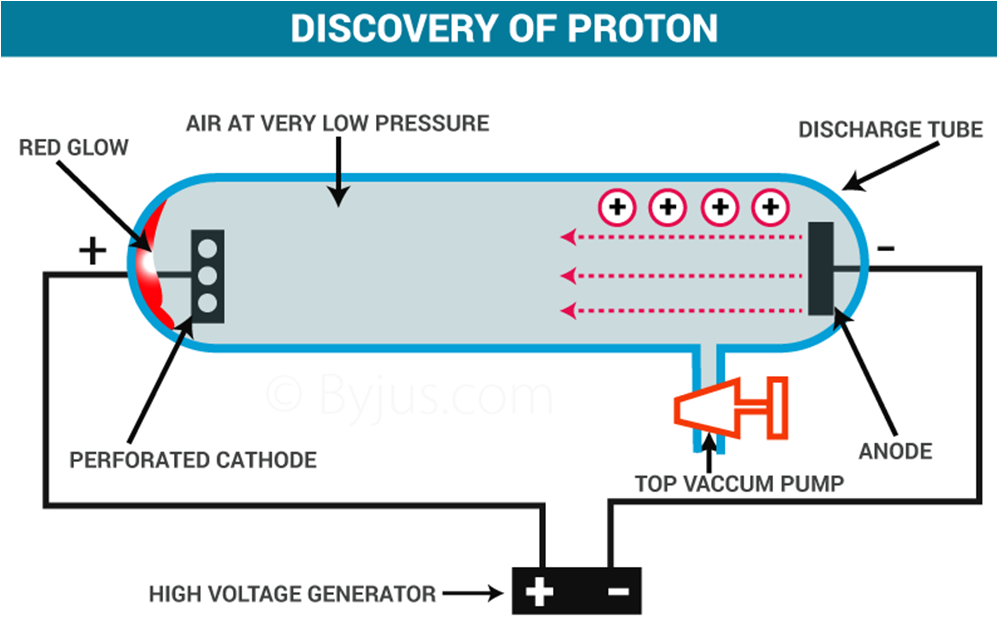
Discovery of Protons – Anode Rays/Canal Rays (By E. Goldstein)
→ E. Goldstein by his famous anode rays/canal rays experiment was able to detect presence of positively charged particles called protons in the atom.
Facts about Protons
→ Charge on proton = + 1.6 × 10-19 C
→ Mass of proton = 1.673 × 10 -24 gm
i.e., Mass of proton ≅ 1840 × Mass of electron
3. Discovery of Neutrons
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Discovery of Neutrons (By J. Chadwick)
→ J. Chadwick bombarded lighter elements (like lithium, boron etc.) with α-particles and observes
emission of new particles having zero charge but having mass equal to that of proton.
→ These particles were called ‘Neutron’ i.e., neutral particle of the atom.
→ Neutron are absent in Protium isotope of hydrogen atom.(1H1)
→ Since, mass of electrons are negligible as compared to that of proton and neutrons hence, sum
of masses of protons and neutrons in an atom will compose its atomic mass.
4. Some Important Defintion
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Atomic Models
→ From the knowledge of existence of subatomic particles like electron, proton and neutron in
atom, various atomic models were proposed by different scientists.
• Some of the atomic models:
(i) Thomson’s Model of Atom
(ii) Rutherford’s Model of Atom
(iii) Bohr’s Model of Atom
→ The most trusted and scientifically established model of atom which is adopted these days
‘Quantum Mechanical Model of Atom’. It will be dealt in higher classes.
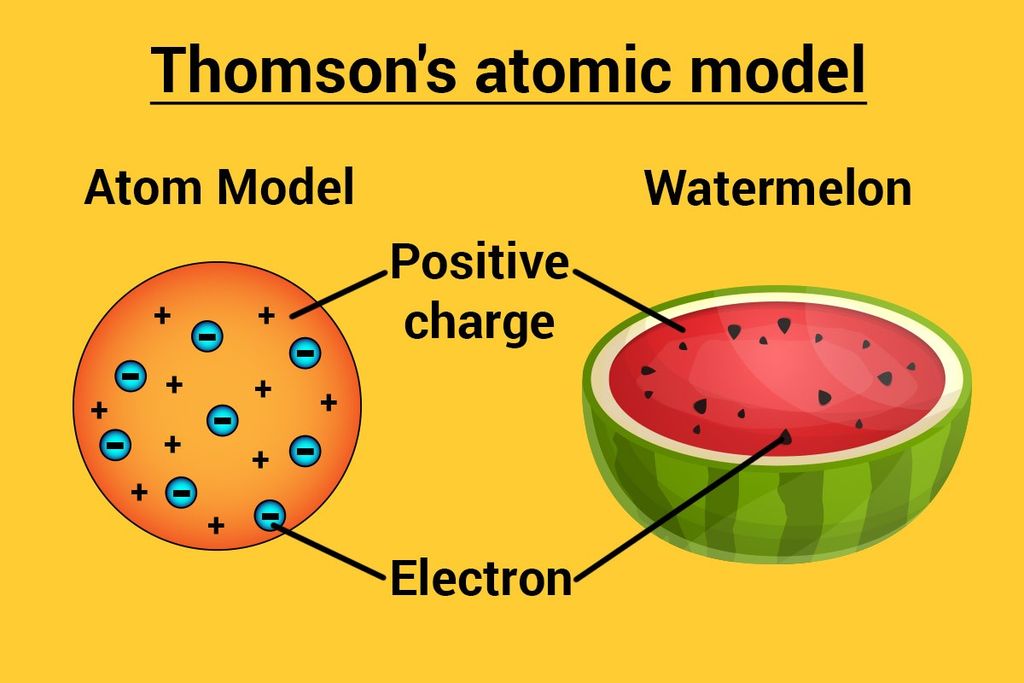
Thomson’s Atomic Model
→ This model is often called the ‘Water Melon Model’.
→ In this model, Thomson predicted the presence of electrons inside positive sphere (made up
protons), just same as seeds of watermelon are embedded in red edible part of watermelon.
→ Although this model explained neutrality of atom but couldn’t able to explain other scientific
experiments conducted on atom. Hence it was discarded.
5. Atomic Number And Mass Number
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Rutherford’s Atomic Model
→ In his famous ‘α-ray Scattering Experiment’, Rutherford bombarded α-ray (Helium nucleus 2 H
upon thin gold foil.
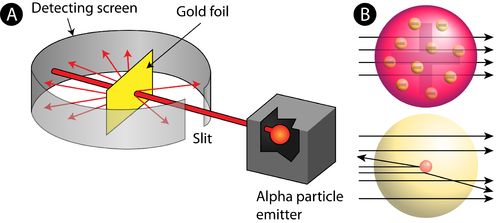
• Observations made by Rutherford in his experiment:
(i) Most of α-particles passed through gold foil undeflected.
(ii) Some of the α-particles deflected by foil by small angles.
(iii) One out of every 12000 particles appeared to rebound.
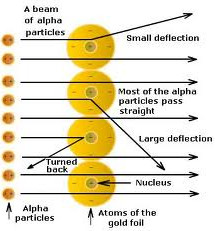
• Conclusions made by Rutherford:
(i) Atom consists of predominantly empty space as most of α-particles passed through gold foil undeflected.
(ii) Atom contains centrally placed positively charged nucleus (carrying positively charged particles), because few α-particles suffered deflected and very few i.e., one in 12000 bounced back.
(iii) Since a minute fraction of α-particles suffered deflections and very few bounced back, this
to conclusion that most of the space an atom is empty and the space occupied by nucleus is negligible compared to this empty space.
→ Size of nucleus was about 10-5 times that of size of atom.
(iv) Whole of the atomic mass concentrated in the nucleus.
• Features of Rutherford proposed model of atom:
(i) There is positively placed nucleus in an atom. Nearly all the mass resides in nucleus (Proton + Neutron).
(ii) Electrons revolves round the nucleus in well defined orbits.
(iii) Size of nucleus is very small compared to the size of atom.
• Drawbacks of Rutherford’s Model (Unstability of Atom)
→ According to Rutherford, electrons revolve round the nucleus in well-defined orbits, but elec
being charged particles will lose their energy and finally will fall into the nucleus.
→ This will make atom highly unstable.
→ This was the major drawback of Rutherford which was unexplained by him.
→ To overcome drawbacks of Rutherford’s Model, Neil Bohr in 1912 proposed modified model of
structure of atom.
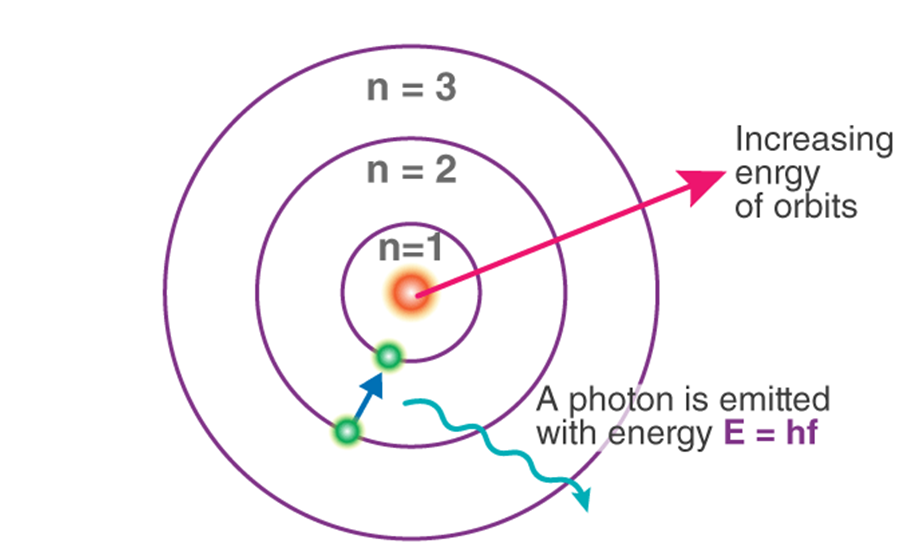
Assumption made by Neil Bohr
→ Only certain special orbits known as discrete orbits of electrons are allowed inside the atom
→ While revolving in discrete orbits, the electrons do not radiate energy.
→ Energy is emitted or absorbed by an atom only when an electron moves from one orbit to another.
6. Isotopes
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Atomic Number
→ The total number of proton lying in the nucleus of any atom is called the atomic number.
→ An atomic number is the identity of an atom, changing atomic number means changing the atom.
→ Atomic number is denoted by ‘Z’.
→ Atomic number = no. of protons or a neutral atom, no. of protons and electrons are equal.
Mass Number
→ It is the sum of total number of protons and no. of neutrons lying in the nucleus of an atom.
→ It is denoted by ‘A’.
→ Mass number = no. of protons + no. of neutrons
→ Representation of an atom:
zXA
(X= symbol of an element)
Example: Calculate number of protons, electrons and neutrons for 17Cl35 or 17Cl37
Sol. Since Cl is neutral,
No. of electrons = no. of protons = 17
Mass no. of Cl = 35
No. of neutrons = 35 - 17 =18
Distribution Of Electrons In Various Shells
→ The distribution of electrons in various shells is done in accordance to ‘Bohr-Bury Scheme’.
Bohr-Bury Scheme
(i) The filling of electrons in an atom is done in accordance to ‘2n2’, where ‘n’ is the number of shell and ‘2n2 ’ represents the total number of electrons that can be accommodated in that particular shell.
→ Maximum number of electrons that can be filled in particular shell.
If n = 1, i.e., K = shell, 2n2= 2×1 2 = 2 electrons
If n = 2, i.e., L = shell, 2n2 = 2×2 2 = 8 electrons
If n = 3, i.e., M = shell, 2n2 = 2×3 2 = 18 electrons
If n = 4, i.e., N = shell, 2n2 = 2×4 2 = 32 electrons
(ii) The outermost shell can’t hold more than 8 electrons, while second last shell can’t have more than 18 electrons, even though they may have capacity to hold more electrons.
Example: ‘Ca 20 ’, the electron distribution will be :
Ca 20 = 2(K), 8(L), 8(M), 2(N)
→ But Ca 20 = 2, 8, 10 is wrong although ‘M’ shell can contain upto 18 electrons.
(iii) The outermost shell can’t hold more than 2 electrons and the penultimate shell can’t hold more than 8 electrons unless the preceding inner shell (antepenultimate shell) is filled completely obey ‘2n2 ’ rule.
(i) K(19) = 2, 8, 8, 1
(ii) Al (13) = 2, 8, 3
(iii) F (9) = 2, 7
(iv) Ne (10) = 2, 8
(v) Na (11) = 2, 8, 1
Valence Shell and Valence Electrons
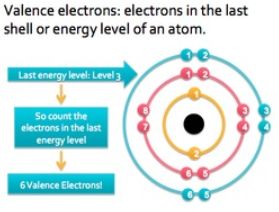
→ From Bohr-Bury sequence, we know that maximum number of electrons which can be accommodated in outermost shell is 8.
→ Every element has an urge to have 8 electrons in its outermost shell, in achieving 8 electrons atom can either gain electrons or loose electrons.
→ The number of electrons lost or gained by an element in achieving 8 electrons in its outermost shell will be called its Valency.
→ For elements like H, He, Li, Be and B, these elements lose their outermost electron to achieve 2 electrons in their outermost shell.
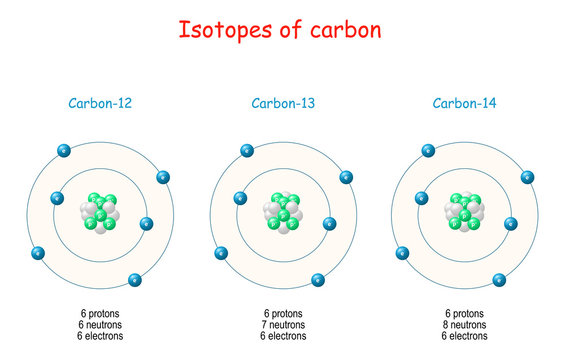
→ Isotopes are atoms of same elements having same atomic number and different mass number.
Example: Chlorine has two isotopes of mass numbers 35 and 37 respectively.
17Cl35 , 17Cl37
Uses of isotopes
(i) Uranium isotope is used as fuel in nuclear rector.
(ii) Isotope of cobalt is useful in treatment of cancer.
(iii) An isotope of iodine is used in the treatment of goitre.
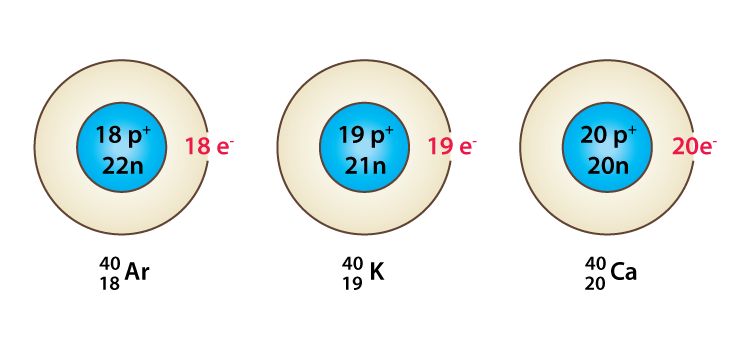
Isobars
→ Isobars are the atoms of those elements which have the same mass number but different atomic
numbers are called isobars.
→ 20Ca40 and 18Ar40 have same mass number and different atomic number. 11Na24 and 12Mg24
another examples.

 Science Made Easy
Science Made Easy
 ACERISE INDIA
ACERISE INDIA
