PHYSICAL NATURE OF MATTER
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Physical Nature of Matter
→ Matter is made up of particles. All matter constitute of very small particles. These small particles are called atoms.
→ These particles of matter are too small so they cannot be seen by naked eyes or simple microscope.
→ Particles of matter are continuously moving as they posses kinetic energy, with the increase in temperature the kinetic energy of particles also increases so particle moves faster.
1. Matter and its Classification
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
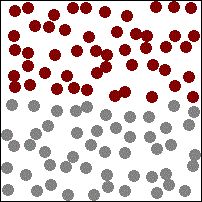
Diffusion
→ Brownian Motion: The zig-zag or random path travelled by the particles of matter is called Brownian motion.
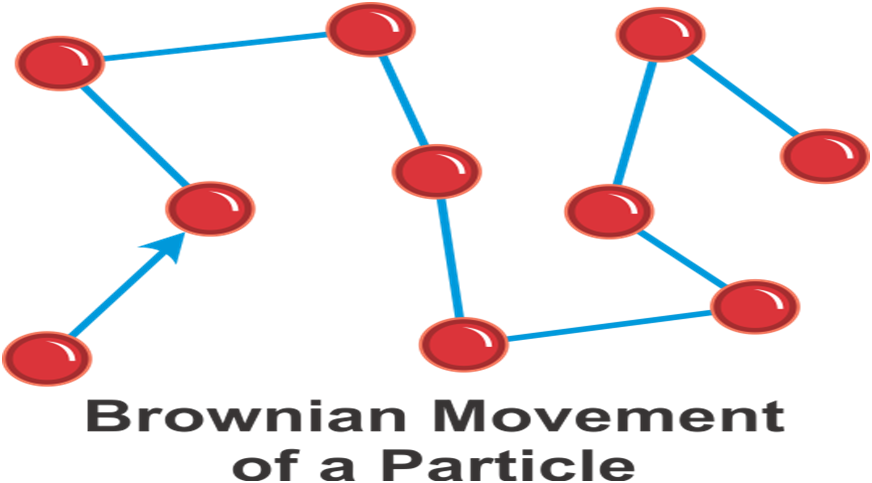
→ Intermixing of particles of two different types of matter on their own is called diffusion.
The rate of diffusion increases on increasing the temperature of the diffusing substance (by heating).
Example - The colour of ink spreading in water due to diffusion of particles of water.

2. Molecular Theory of Matter
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Characteristics of Particles of Matter
• Particles of matter have space between them
→ Gas can be compressed a lot because of the space between their particles.
Activity:- take some water in a beaker and note its level. Dissolve some salt or sugar in it with the help of a glass rod. The salt dissolves in the water but the level of water dose not change. This is because the particles of salt get the space between the particles of water.
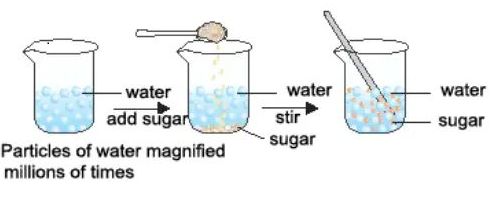
→ Additionally you would notice that there is no rise of water level takes place when one or two teaspoon of sugar/salt is added into a glass of water, this is because sugar/salt particles get adjusted in the space between the particles of water and no rise in the water level comes in result.
• Particles of matter attract each other because of force of attraction.
Activity:- Take an iron nail, a piece of chalk and rubber band. Try breaking them hummering, cutting or stretching. It is more easier to break the chalk, less easier to break the rubber band and difficult to break the iron nail. this is because the particles in the iron nail are held together with greater force than in the rubber band or challk.
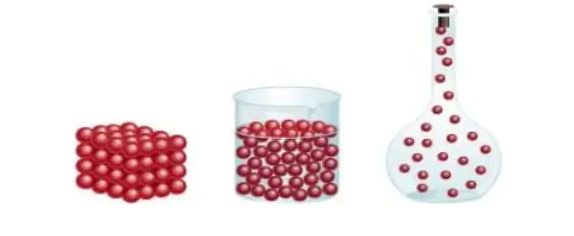
→ Force of attraction between particles of matter keeps the particles bonded together. Therefore attraction between particles of solid is greatest, between particles of liquid is moderate and between particles of gas is lowest.
→ Because of the lowest force of attraction between the particles of gas we can move our hand through
air easily. To move our hand in liquid, such as water, we have to apply some force, but a solid such as wood, we cannot move our hand.
→This is because the force of attraction between particles of gas is almost negligible, in liquid the forces of attraction is moderate but it is greatest in solid.
→ The force of attraction between particles of solid, liquid and gas can be arranged in decreases in order as follows:
Solid > Liquid > Gas
• Particles of matter are continuously moving –
Activity :- Take some water in a beaker and put a drop of blue or red ink slowly along the sides of the beaker. Leave it undisturbed for a few hours. The ink spreads evenly throughout the water due to the movement of the particles of water and ink.
The intermixing of two or more different types of matter on their own is called diffusion.
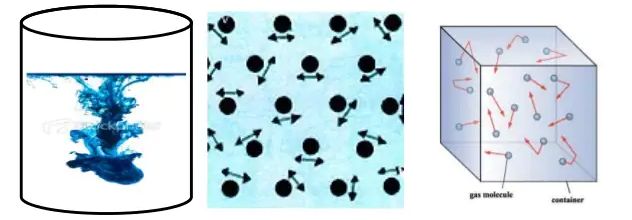
• Matter is made up of small particles –
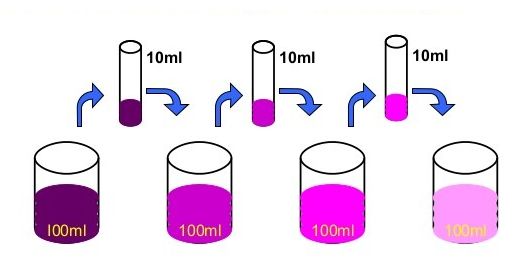
Activity :- Dissolve 2 —3 crystals of potassium permanganate in I OOml of water in a beaker. Take lOmI of this solution and dissolve in lOOmI of water. Take lOmI of this solution and dissolve In lOOmI of water. Repeat this process 5— 6 times. This shows that a few crystals of potassium permanganate can colour a large volume of water because there are millions of tiny particles in each crystal
Classification of states of matter
States of Matter
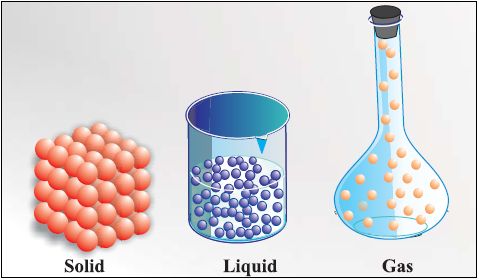
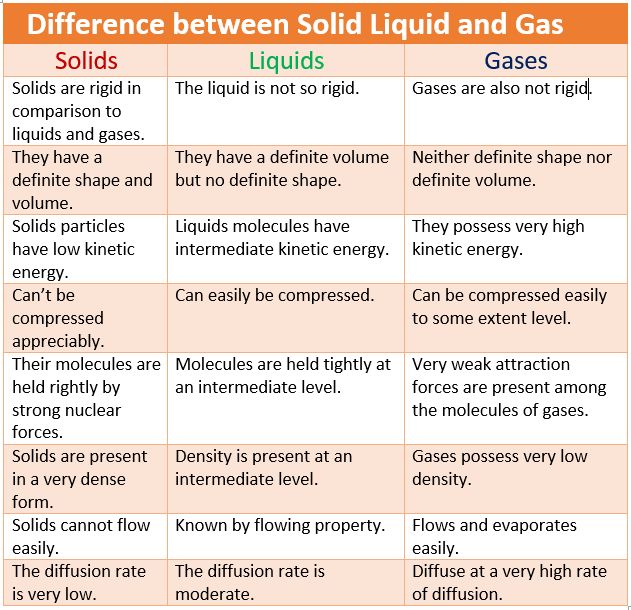
• Solid State
→ The space between the particles is very less.
→ The force of attraction between the particles is strong. Thus, particles in a solid are closely packed.
→ Solids maintain their shape even when they are subjected to external force i.e. they are rigid
→ Solids cannot be compressed.
→ The kinetic energy of the particles is very less and so solids have an orderly arrangement of particles. Therefore, solids have a fixed shape and volume.
• Liquid State
→ The space between the particles is slightly more as compared to solids, but still very less as compared to gases. The particles of a liquid can slip and slide over each other.
→ The force of attraction between the particles is strong enough to hold the particles together not strong enough to hold the particles in a fixed position.
→ Liquids do not have a fixed shape but have a fixed volume. Liquids take up the shape of the container in which they are poured.
→ The kinetic energy of the particles is more than that of solids. Thus, liquids have a disorderly arrangement of particles compared solids.
→ Liquids cannot be compressed much. The compressibility of liquids is almost negligible.
• Gaseous State
→ The particles are much farther apart from one another as compared to solids and liquids.They have a very disorderly arrangement of particles compared to the solids and liquids.
→ The force of attraction between the particles is negligible, hence particles of a gas move free in all the directions.Gases thus can mix or diffuse into other gases.
→ The particles of a gas have maximum kinetic energy. They move with high speed in all directions and can exert pressure on the walls of its container.
→ Gases neither have a definite shape nor a definite volume.They fill up the container completely
→ Gases can be compressed easily. Example: the LPG cylinders used at home and the CNG cylinder used in vehicles.
3. Plasma and BEC State, Temperature
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Change of State of Matter

→ The phenomenon of change from one state of matter to another, and then back to the original state is called the interconversion of states of matter.
→ Matter Can Change its State. Water can exist in three states of matter:
• Solid as ice
• Liquid as water
• Gas as water vapour
Effect of Temperature
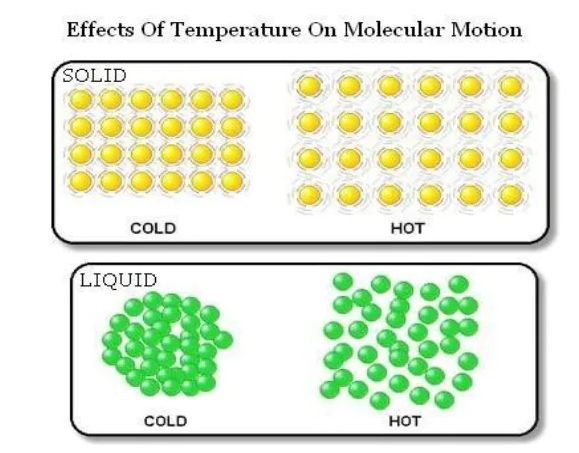
→ On increasing the temperature of solids, the kinetic energy of the particles increases which overcomes the forces of attraction between the particles thereby solid melts and is converted into liquid.
→ The temperature at which a solid melts to become a liquid at the atmospheric pressure is called its
melting point.
The melting point of ice is 273.16 K.
The process of melting, that is, change of solid state into liquid state is also known as fusion.
4. Interconversion of States of Matter
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
• Latent heat
The hidden heat which breaks the force of attraction between the molecules is known as the latent heat. Since, the heat energy is hidden in the bulk of the matter, it is called latent heat.
• Latent heat of fusion
→ The heat energy required to convert 1 kilogram of a solid into liquid at atmospheric pressure,at melting point, is known as the latent heat of fusion.
→ The temperature at which a liquid starts boiling, at atmospheric pressure, is called its boiling point.
• Latent heat of vaporisation
→ The heat energy required to convert 1 kilogram of liquid into gas, at atmospheric pressure, at
boiling point, is known as the latent heat of vaporisation
→ The process, in which a gas, on cooling, turns into a liquid at a specific temperature is called
condensation or liquefaction.
Formation of clouds is due to the condensation of water vapour from the Earth’s surface.
→ The temperature at which the state of a substance changes from a liquid to a solid is called freezing point of that substance.
→ When a solid melts, its temperature remains the same because heat gets used up in changing the state by overcoming the forces of attraction between the particles. It is considered that it gets hidden into the contents of the beaker and is known as the latent heat.
→ Water vapour at 373 K have more energy than water at the same temperature because particles in steam have absorbed extra energy in the form of latent heat of vaporisation.
5. Evaporation
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
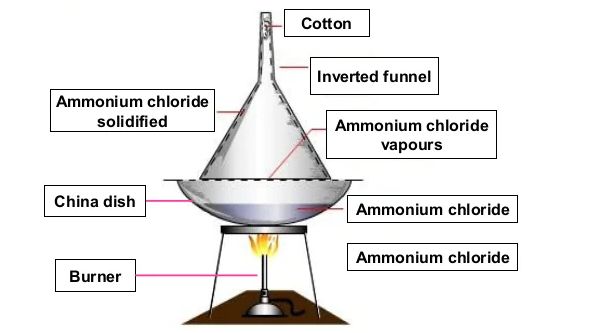
Sublimation –
→ The change of state of a substance directly from a solid to gas, without changing into the liquid
state (or vice versa) is called sublimation.
Examples – Naphthalene balls, Camphor, Ammonium chloride, iodine etc.
EFFECT OF CHANGE OF PRESSURE
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Effect of change of pressure
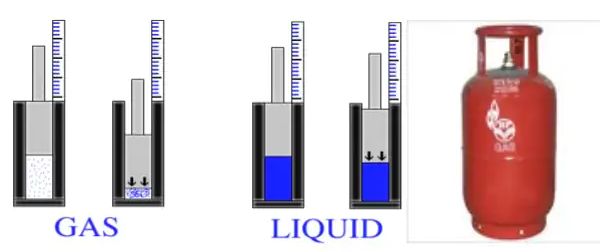
→ Gases can be liquefied by applying pressure and reducing the temperature. When a high pressure is applied to a gas, it gets compressed and if the temperature is lowered, the gas is liquefied.
→ Solid CO2 gets converted directly to gaseous state on decrease of pressure to 1 atmosphere without coming into liquid state. This is the reason that solid carbon dioxide is also known as dry ice
EVAPORATION
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Evaporation
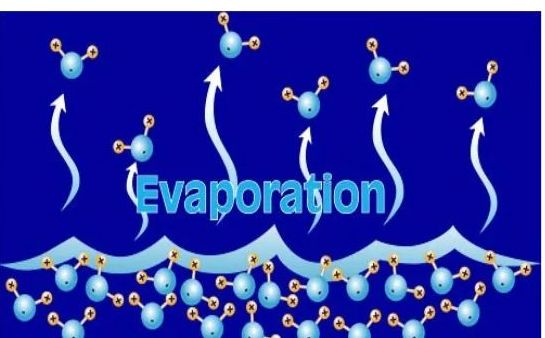
→ The process of conversion of a substance from the liquid state to the gaseous state at any temperature below its boiling point is called evaporation or vaporisation.
→ Evaporation is a surface phenomenon.
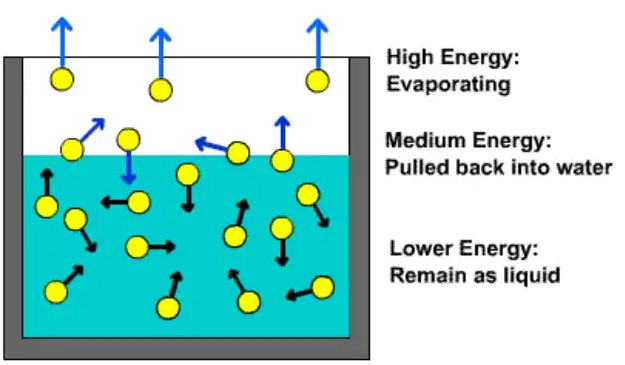
• Factors affecting the rate of evaporation
→ Surface Area – The rate of evaporation increases on increasing the surface area of the liquid.
→ Temperature - The rate of evaporation increases with an increase in temperature.
→ Humidity - Decrease in the humidity increases the rate of evaporation.
→ Wind Speed - An increase in the wind speed increases the rate of evaporation.
• Evaporation causes cooling
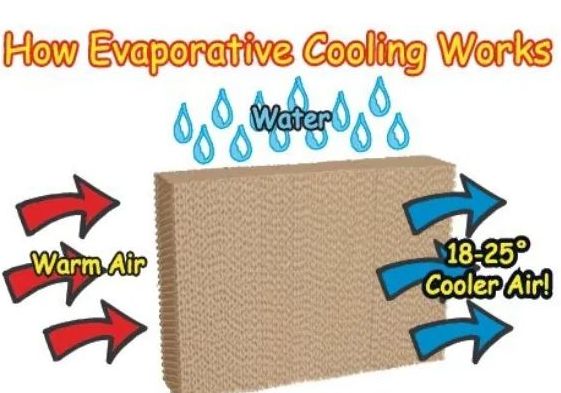
→ The particles of liquid absorb energy from the surrounding to regain the energy lost during
evaporation. This absorption of energy from the surroundings make the surroundings cold.
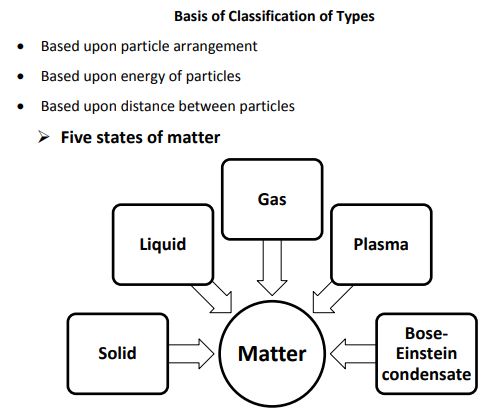
→ Lately, scientists are talking about five states of matter or five phases of matter. These are-so
liquids, gases, plasmas and the Bose–Einstein condensate.
OTHERS STATES OF MATTER
- Books Name
- Science Made Easy Science Book
- Publication
- Science Made Easy
- Course
- CBSE Class 9
- Subject
- Science
Plasma
The state consists of super energetic and super excited particles. These particles are in the form
ionised gases. The fluorescent tube and neon sign bulbs consist of plasma.
Bose-Einstein Condensate
→ Indian physicist Satyendra Nath Bose made a study regarding the fifth state of matter. Base
his study, Albert Einstein predicted a fifth state of matter called the Bose-Einstein Condensate.
→ The Bose-Einstein Condensate or BEC is formed by cooling a gas of extremely low density to
low temperatures.
Conversion of Temperature –
→ Kelvin is the SI unit of temperature, C = 273.16
For convenience, we take 0° C = 273 K
after rounding off the decimal. To change a temperature
on the Kelvin scale to the Celsius scale you have to subtract 273 from the given temperature, and to convert a temperature on the
Celsius scale to the Kelvin scale you have to add 273 to the given temperature.
→ Atmosphere (atm) is a unit of measuring pressure exerted by a gas. The unit of pressure is Pascal.
(Pa): 1 atmosphere = 101325 Pa.
The pressure of air in atmosphere is called atmospheric pressure & atmospheric pressure at sea level is 1 atmosphere, and is taken as the normal atmospheric
pressure.
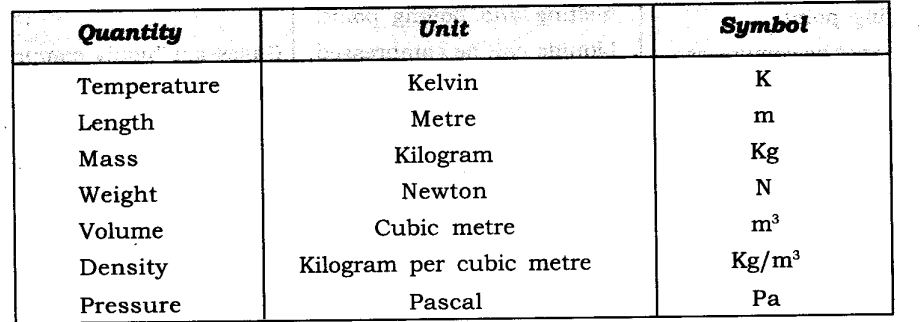
Some measurable quantities and its units

 EduMple Learning
EduMple Learning
 ACERISE INDIA
ACERISE INDIA
