1. Chemical Equations
- Books Name
- Chemistry Class 10 NCERT based
- Publication
- Grow Career Publication
- Course
- CBSE Class 10
- Subject
- Chemistry
Chemical Reactions and Equations: Balanced and unbalanced chemical equations and balancing of chemical equations.Consider the following situations of daily life and think what happens when , In all the above situations, the nature and the identity of the initial substance have somewhat changed. We have already learnt about physical and chemical changes of matter in our previous classes. Whenever a chemical change occurs, we can say that a chemical reaction has taken place
- Milk is left at room temperature during summers.
- An iron tawa/pan/nail is left exposed to humid atmosphere.
- Grapes get fermented.
- Food is cooked.
- Food gets digested in our body.
- We respire.
Chemical Reaction: The transformation of chemical substance into another chemical substance is known as Chemical Reaction. For example: Rusting of iron, the setting of milk into curd, digestion of food, respiration, etc.
Example: The burning of magnesium in the air to form magnesium oxide is an example of a chemical reaction.
2Mg(s) + O2(g) △→ 2MgO(s)
Before burning in air, the magnesium ribbon is cleaned by rubbing with sandpaper . This is done to remove the protective layer of basic magnesium carbonate from the surface of the magnesium ribbon. Reactant: Substances which take part in a chemical reaction are called reactants. Example: Mg and O2.
Product: New substance formed after a chemical reaction is called a product.
Example: MgO.
Chemical Reaction's Characteristics
1. Change in temperature: The chemical reaction between quick lime water to form slaked lime is characterized by a change in temperature (which is a rise in temperature).
2. Change in state of substance: The combustion reaction of candle wax is characterised by a change in state from solid to liquid and gas (because the wax is a solid, water formed by the combustion of wax is a liquid at room temperature.
3. Formation of precipitate: The chemical reaction between sulphuric acid and barium chloride solution is characterised by the formation of a white precipitate of barium sulphate.
BaCl2(aq) + H2SO4(aq) ----→ BaSO4(s) (ppt) + 2HCl(aq)
4. Evolution of gas: The chemical reaction between zinc and dilute sulphuric acid is characterised by the evolution of hydrogen gas.
Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g)
Chemical Equations - The chemical equation of the reaction is the representation of a chemical change in terms of symbols and formulae of the reactants and products.
Example: Potassium Hydrochloric Potassium Manganese Water Chlorine permanganate acid → chloride chloride
(a) Balanced Chemical Equation: A balanced chemical equation has the number of atoms of each element equal on both sides.
Example: Zn + H2SO4 → ZnSO4 + H2
In this equation, numbers of zinc, hydrogen and sulphate are equal on both sides, so it is a Balanced Chemical Equation.
(b) Unbalanced Chemical Equation: If the number of atoms of each element in reactants is not equal to the number of atoms of each element present in the product, then the chemical equation is called Unbalanced Chemical Equation.
Example: Fe + H2O → Fe3O4 + H2
Balancing a Chemical Equation: To balance the given or any chemical equation, follow these steps:
Fe + H2O → Fe3O4 + H2
Table as shown here.
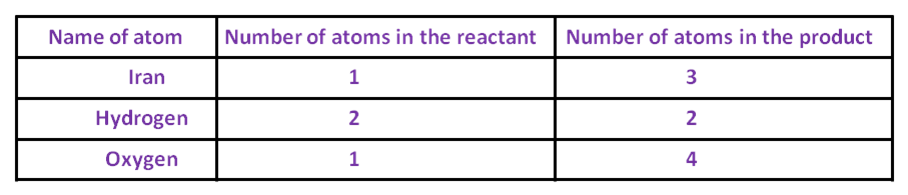
Fe + 4 × H2O → Fe3O4 + H2To balance the oxygen, one needs to multiply the oxygen on the LHS by 4, so that, the number of oxygen atoms becomes equal on both sides.
Now, the number of hydrogen atoms becomes 8 on the LHS, which is more than that on the RHS. To balance it, one needs to multiply the hydrogen on the RHS by 4.
Fe + 4 × H2O → Fe3O4 + 4 × H2
After that, the number of oxygen and hydrogen atoms becomes equal on both sides. The number of iron is one on the LHS, while it is three on the RHS. To balance it, multiply the iron on the LHS by 3.
3 × Fe + 4 × H2O → Fe3O4 + 4 × H2
Now the number of atoms of each element becomes equal on both sides. Thus, this equation becomes a balanced equation.
After balancing, the above equation can be written as follows:
3Fe + 4H2O → Fe3O4 + 4H2.
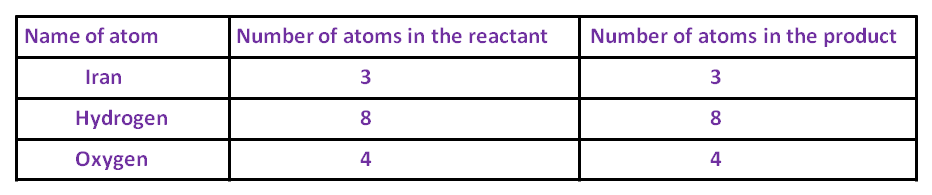
1. Chemical Equations
- Books Name
- Chemistry Book based on NCERT
- Publication
- PRIDE LEARNING PUBLICATION
- Course
- CBSE Class 10
- Subject
- Chemistry
CHAPTER  1
1
CHEMICAL REACTIONS AND EQUATIONS
* CHEMICAL EQUATIONS
CHEMICAL REACTIONS:- The process in which two or more substance combine with each other to form new substances with new properties is called chemical reaction.
There are two parts of a chemical reactions :-
(i) Reactants:- The substances which take part in a chemical reaction are known as reactants.
(ii) Products:- The new substances formed during a chemical reaction are known as products.
There are 5 ways to tell if a chemical reaction has occurred.
- Change in state.
- Change in colour.
- Change in temperature.
- Evolution of a gas.
- Formation of precipitate.
Chemical reaction in everyday life:-
- Digestion of food.
- Respiration.
- Rusting of iron.
- Formation of curd.
- Burning of magnesium ribbon.
Chemical Equations:- A chemical equation is a written representation of a chemical reaction.
The representation of chemical reaction using symbols and formulae of the substances is called chemical equation.
A + B
Reactants Products
n this equation, A and B are called reactants and C and D are called the products. The arrow shows the direction of the chemical reaction. The necessary condition such as temperature, pressure or any catalyst should be written on arrow between reactants and products.
E.g. Magnesium is burnt in air to form magnesium oxide.
(i) Word equation for above reaction would be -
Magnesium + oxygen
( Reactants ) ( Product )
Skeletal equation for above reaction would be -
Mg +
BALANCING CHEMICAL EQUATIONS:-
- LAW OF CONSERVATION OF MASS :- Mass can neither be created nor be destroyed in a chemical reaction.
- So number of elements involved in chemical reaction should remain same at reactant and products side.
For Example ,
Zn +
(Zinc) ( Sulphuric Acid) (Zinc Sulphate) ( hydrogen)
Let us check the number of atoms of different elements on both sides of the arrow .

As the number of atoms of each element is same on both sides of arrow. This is a balanced chemical equation.
Let us take another example :-
Fe +
STEP 1 :- Write a chemical equation.
Fe +

STEP 3 :_ Select the element which has the maximum number of atoms . now equalize the number of atoms by putting coefficient in front of it.
Fe + 4
STEP 4 :- Fe and H atoms are still not balanced choose any elements now to balance. To equalize the number of H atoms,
Fe + 4
STEP 5 :- Now, take Fe and equalize the number of Fe atoms.
3 Fe + 4  F
F
Now all the atoms of elements are equal on both sides.
STEP 6 :- To make the chemical equation more information ,write the physical states of reactants and products.
Solid state = (s)
Liquid state = (l)
Gaseous State = (g)
Aqueous state = (aq)
3 Fe (S) + 4
STEP 7:- Write necessary conditions of temperature pressure or catalyst on above or below arrow.
For Example:-
![]()
2. Types of Chemical Reactions
- Books Name
- Chemistry Class 10 NCERT based
- Publication
- Grow Career Publication
- Course
- CBSE Class 10
- Subject
- Chemistry
Types of Chemical Reactions: Combination Reaction, Decomposition Reaction, Displacement Reaction, Double Displacement Reaction, Neutralization Reactions, Exothermic – Endothermic Reactions and Oxidation-Reduction Reactions.
A. Combination or Synthesis Reactions:
The reactions in which two or more chemicals combine to generate a single new compound.
Types of Combination reactions:
I. Combination of two elements to form a compound
● Burning of hydrogen in air or oxygen to produce water.
H²(hydrogen) + O²( oxygen) → H²O(water) + O²( oxygen)
II. Combination Reactions involving an Element and a Compound
● Burning of carbon monoxide in oxygen to form carbon dioxide.
2CO (carbon monoxide) + O2(Oxygen) → 2CO2(Carbon dioxide)
III. Combination Reactions involving Two Compounds
● Combination of ammonia and hydrogen chloride to produce ammonium chloride.
NH³( Ammonia) + HCl(Hydrogen chloride) → NH⁴Cl³( Ammonium chloride)
B . Decomposition Reaction: Reactions in which one compound decomposes in two or more compounds or elements are known as Decomposition Reaction. A decomposition reaction is just the opposite of combination reaction.
A general decomposition reaction can be represented as follows :
AB → A + B
Examples:
When calcium carbonate is heated, it decomposes into calcium oxide and carbon dioxide.
CaCO3(s) heat−→− CaO(s) + CO2(g)
Calcium carbonate → Calcium oxide + Carbon dioxide
When ferric hydroxide is heated, it decomposes into ferric oxide and water
2Fe(OH)3(s) △→ Fe2O3(s) + 3H2O(l)
Thermal Decomposition: The decomposition of a substance on heating is known as Thermal Decomposition.
Example: 2Pb(NO3)2(s) heat−→− 2PbO(s) + 4NO2(g) + O2(g)
Electrolytic Decomposition: Reactions in which compounds decompose into simpler compounds because of passing of electricity, are known as Electrolytic Decomposition. This is also known as Electrolysis.
Example: When electricity is passed in water, it decomposes into hydrogen and oxygen.
2H2O(l) Undefined control sequence \xrightarrow 2H2(g) + O2(g)
Photolysis or Photo Decomposition Reaction: Reactions in which a compound decomposes because of sunlight are known as Photolysis or Photo Decomposition Reaction.
Example: When silver chloride is put in sunlight, it decomposes into silver metal and chlorine gas.
2AgCl(s) (white) Sunlight−→−−−−− 2Ag(s) (grey) + Cl2(g)
Photographic paper has a coat of silver chloride, which turns into grey when exposed to sunlight. It happens because silver chloride is colourless while silver is a grey metal.
C. Displacement Reaction: The chemical reactions in which a more reactive element displaces a less reactive element from a compound is known as Displacement Reactions. Displacement reactions are also known as Substitution Reaction or Single Displacement/ replacement reactions.
A general displacement reaction can be represented by using a chemical equation as follows :
A + BC → AC + B
Displacement reaction takes place only when ‘A’ is more reactive than B. If ‘B’ is more reactive than ‘A’, then ‘A’ will not displace ‘C’ from ‘BC’ and reaction will not be taking place.
Examples:
When zinc reacts with hydrochloric acid, it gives hydrogen gas and zinc chloride.
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
When zinc reacts with copper sulphate, it forms zinc sulphate and copper metal.
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
D. Double Displacement Reaction: Reactions in which ions are exchanged between two reactants forming new compounds are called Double Displacement Reactions.
AB + CD → AC + BD
Examples:
When the solution of barium chloride reacts with the solution of sodium sulphate, white precipitate of barium sulphate is formed along with sodium chloride.
BaCl2(aq) + Na2SO4(aq) → BaSO4(s) (Precipitate) + 2NaCl(aq)
When sodium hydroxide (a base) reacts with hydrochloric acid, sodium chloride and water are formed.
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
Note: Double Displacement Reaction, in which precipitate is formed, is also known as precipitation reaction. Neutralisation reactions are also examples of double displacement reaction.
Precipitation Reaction: The reaction in which precipitate is formed by the mixing of the aqueous solution of two salts is called Precipitation Reaction.
Example:
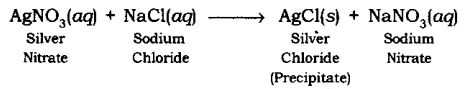
Neutralization Reaction: The reaction in which an acid reacts with a base to form salt and water by an exchange of ions is called Neutralization Reaction.
Example:
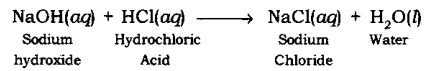
E. Oxidation and Reduction Reactions:
Oxidation: Addition of oxygen or non-metallic element or removal of hydrogen or metallic element from a compound is known as Oxidation.
Elements or compounds in which oxygen or non-metallic element is added or hydrogen or metallic element is removed are called to be Oxidized.
Reduction: Addition of hydrogen or metallic element or removal of oxygen or non-metallic element from a compound is called Reduction.
The compound or element which goes under reduction in called to be Reduced.
Oxidation and Reduction take place together. For example
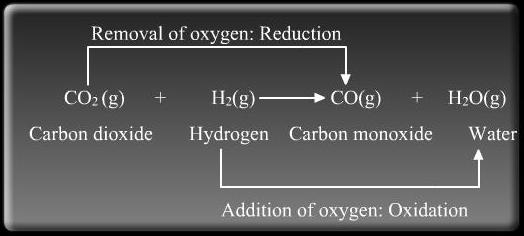
Oxidizing agent:
- The substance which gives oxygen for oxidation is called an Oxidizing agent.
- The substance which removes hydrogen is also called an Oxidizing agent.
Reducing agent:
- The substance which gives hydrogen for reduction is called a Reducing agent.
- The substance which removes oxygen is also called a Reducing agent.
The reaction in which oxidation and reduction both take place simultaneously is called Redox reaction.
When copper oxide is heated with hydrogen, then copper metal and hydrogen are formed.
CuO + H2 → Cu + H2O
(i) In this reaction, CuO is changing into Cu. Oxygen is being removed from copper oxide. Removal of oxygen from a substance is called Reduction, so copper oxide is being reduced to copper.
(ii) In this reaction, H2 is changing to H2O. Oxygen is being added to hydrogen. Addition of oxygen to a substance is called Oxidation, so hydrogen is being oxidised to water.
- The substance which gets oxidised is the reducing agent.
- The substance which gets reduced is the oxidizing agent
F. Exothermic and Endothermic Reactions:
Exothermic Reaction: Reaction which produces energy is called Exothermic Reaction. Most of the decomposition reactions are exothermic.
Example:
Respiration is a decomposition reaction in which energy is released.
![]()
When quick lime (CaO) is added to water, it releases energy.
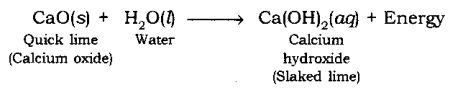
Endothermic Reaction: A chemical reaction in which heat energy is absorbed is called Endothermic Reaction.
Example: Decomposition of calcium carbonate.

2. Types of Chemical Reactions
- Books Name
- Chemistry Book based on NCERT
- Publication
- PRIDE LEARNING PUBLICATION
- Course
- CBSE Class 10
- Subject
- Chemistry
TYPE OF CHEMICAL REACTION
There are five type of chemical reactions.
COMBINATION REACTION:- The reaction in which two or more reaction combine to form a single product is called combination reaction They are represented by equation of the following term.
Reactant product
For Example :-
Burning of coal
C(s) +
(carbon) (oxygen) (carbon dioxide)
Formation of water
2
(Hydrogen) (oxygen) (Water)
Formation of slaked line
CaO (s) +
(calcium oxide) (water) (calcium Hydroxide)/ slaked lime
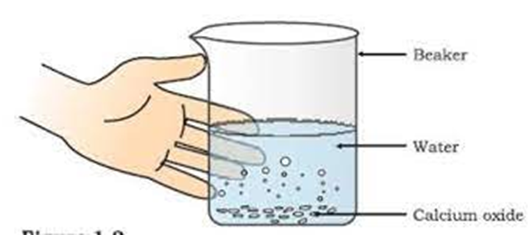
Formation of slaked lime by the reaction of calcium oxide with water.
DECOMPOSITION REACTION :- The reaction I which single compound breaks down to simpler product is called decomposition reaction. They are represented by equation of the following term .
AB
(i) Thermat decomposition :- when decomposition is carried out by heating.
For example
(i) 2 Fe S
(Ferrous sulphate ) (Ferric Oxide)
Green colour Red brown colour
(ii) CaC
(calcium carbonate) ( calcium oxide ) (Carbon dioxide ) Limestone quick lime
(iii) 2Pb (N
(Lead nitrate ) (lead oxide) (Nitrogen dioxide) oxygen
Brown fumes
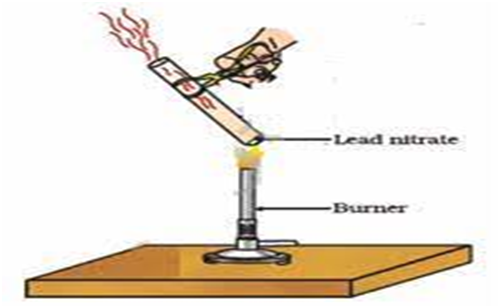
Heating of lead nitrate and emission of nitrogen dioxide
ELECTRONIC DECOMPOSITION :- When decomposition is carried out by passing electricity .
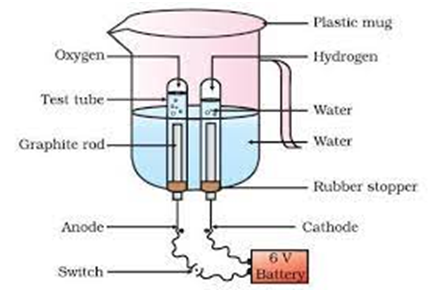
For Example:- (i) Electrolysis of water.
Electric current
2
(Water) (Hydrogen) (Oxygen)
Electrolysis of water is done as follows:-
When decomposition is carried out in presence of sunlight .
For Example:-
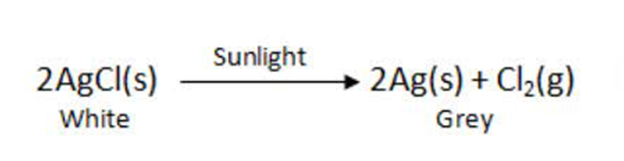
This is why silver chloride turns grey in sunlight because of the decomposition of silver chloride into silvers and chloride by light.
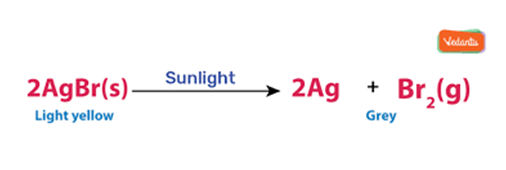
This reaction is used in black and write Photography.
DISPLACEMENT REACTION :- This reaction in which more reactive element displace less reative element from its salt solution is called displacement reaction . they aare represented by equation of the following term.
A + BC
For example :-
(i) Fe (s) + CuS
(Iron ) (copper Sulphate) ( iron Sulphate) ( Copper)
Fe is more reactive than therefore iron (Fe) has displaced copper (Cu) from copper sulphate solution.
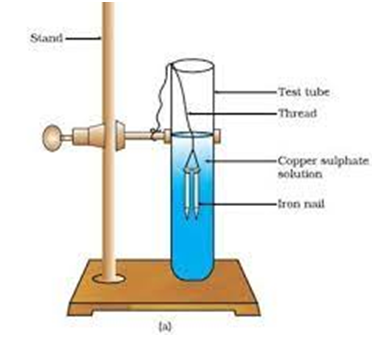
Iron nails dipped in copper sulphate solution.
(ii) Zn(s) + CuS
(Zinc) (copper sulphate) ( Zinc sulphate ) copper
Zinc is more reactive than copper , therefore it displace copper from copper sulphate solution.
(iii) Pb (s) + CuC
(Lead) (copper chloride) ( lead chloride) copper
Lead is more reactive elements than copper, therefore it displaces copper from copper chloride solution.
DOUBLE DISPLACEMENT REACTIVE :- The reaction in which the reactant ions exchange to form new products is called double displacement reaction . they are represented by equation of the following term.
AB + CD
For Example :-
N
(sodium sulphate) (barium chloride) (barium sulphate) (sodium chloride)
White precipitate of BaS
OXIDATION AND REDUCTION :-
OXIDATION
(i) The addition of oxygen to reactant
(ii) the removal of hydrogen from reactant
For Example :-
2 Cu +
(Copper) (oxygen) (copper oxide )
Black substance
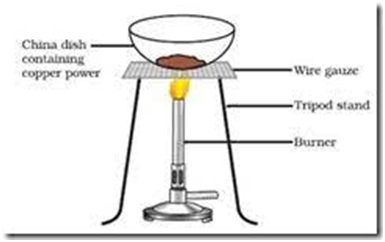
Oxidation of copper to copper oxide
Reduction : - (i) the addition of hydrogen to reactant
(ii) the removal of oxygen from a reactant.
Redox Reactions :- The reaction on written one substance gets oxidisied and other get reduced is known as redox reaction
For example :-
CuO +
In this reaction CuO is reduced to Cu and
ZnO + C
Here, C is oxidized to CO because oxygen is being added and ZnO IS REDUCED TO Zn because O is being removed.
NOTE :
* If a substance loses oxygen during a reactant , it is said to be reduced.
ENDETHERMIC REACTION:- Reaction in which energy is absorded are known as endothermic reaction.
For example :-
CaC
FXOTHERMIC REACTION :- Reaction in which heat is released along with formation of products.
For Example,
C
3. Effects of Oxidation Reactions in Everyday Life
- Books Name
- Chemistry Class 10 NCERT based
- Publication
- Grow Career Publication
- Course
- CBSE Class 10
- Subject
- Chemistry
Effects of Oxidation Reactions in Everyday life: Corrosion and Rancidity.
Corrosion: The process of slow conversion of metals into their undesirable compounds due to their reaction with oxygen, water, acids, gases etc. present in the atmosphere is called Corrosion.

Example: Rusting of iron.
Corrosion of Copper: Copper objects lose their lustre and shine after some time because the surface of these objects acquires a green coating of basic copper carbonate, CuCO3.Cu(OH)2 when exposed to air.
Corrosion of Silver Metal: The surface of silver metal gets tarnished (becomes dull) on exposure to air, due to the formation of a coating of black silver sulphide(Ag2S) on its surface by the action of H2S gas present in the air.
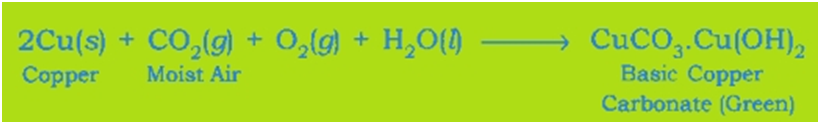

Rancidity: The taste and odour of food materials containing fat and oil changes when they are left exposed to air for a long time. This is called Rancidity. It is caused due to the oxidation of fat and oil present in food materials.
Methods to prevent rancidity:
By adding anti-oxidant.
Vacuum packing.
Replacing air by nitrogen.
Refrigeration of foodstuff.
A. Chemical Reaction: During the chemical reactions, the chemical composition of substances changes or make new substances are formed known as chemical reaction.
B. Chemical Equation: Chemical reactions can be written in chemical equation form which should always be balanced are know as chemical equation.
C. Types of Chemical Reactions:
Combination reaction: A single product is formed from two or more than two reactants.
2Mg + O2 → 2MgO
Decomposition reaction: A single reactant breaks down two or more then two products.
Thermal decomposition:
2Pb(NO2)2 → 2PbO + 4NO2 + O2
Electrolysis:
2H20 → 2H2 + O2
Photochemical reaction:
2AgBr → 2Ag + Br2
Displacement reaction: One element is displaced by another element.this format call di
Zn + CuSO4 → ZnSO4 + Cu
Double displacement reaction: Exchange of ions between reactants.
AgNO3 + NaCl → AgCl + NaNO3
Redox reaction: Both oxidation and reduction take place simultaneously.
CuO + H2 → Cu + H2O
Exothermic reaction: A chemical reaction in which heat energy is evolved.
C + O2 → CO2 (g) + heat
Endothermic reaction: A chemical reaction in which heat energy is absorbed.
ZnCO3 + Heat → ZnO + CO2
5. Reduction: Reaction that shows the loss of oxygen or gain of hydrogen.
ZnO + C → Zn + CO
ZnO is reduced to Zn—reduction. C is oxidized to CO—Oxidation.
Effects of Oxidation Reactions in Our Daily Life:
Corrosion: It is an undesirable change that occurs in metals when they are attacked by moisture, air, acids and bases.
Example, Corrosion (rusting) of Iron: Fe2O3. nH2O (Hydrated iron oxide)
Rancidity: Undesirable change that takes place in oil containing food items due to the oxidation of fatty acids.
Preventive methods of rancidity: Adding antioxidants to the food materials, storing food in the airtight container, flushing out air with nitrogen gas and refrigeration
3. Effects of Oxidation Reactions in Everyday Life
- Books Name
- Chemistry Book based on NCERT
- Publication
- PRIDE LEARNING PUBLICATION
- Course
- CBSE Class 10
- Subject
- Chemistry
EFFORTS OF OXIDATION REACTIONS IN EVERYDAY LIFE
1) COROSION :- When a metal is exposd to moisture ,air, acid eyc. For some time , a layer of hydrated oxide, is formed which e weaknes the metal and hence metal is said to be corroded.
Example of corrosion are:-
- Rusting of iron .
- Black coating on silver
- Green coating on copper.
• Rusting of iron :- when iron is exposed to oxygen in the presence of moisture, reddish brown power is formed.
This is process is knon as rusting of iron.
• Method to prevent corrosion are:-
- Galvanization
- Electroplating
- By putting paints
2) RANCIDITY:- The oxidation of fats and oils when exposed to air is known as rancidity . due to rancidity, bad smell and bad taste of food occurs
Method of prevent rancidity are :-
- By adding antioxidants.
- Refrigeration.
- Replacing air by nitrogen.
- Keeping food in air tight containers.
• Chips manufactures fill bag of chips with nitrogen because it is non reactive gas and it prevent the chips from getting oxidized.

 Grow Career Publication
Grow Career Publication
 PRIDE LEARNING PUBLICATION
PRIDE LEARNING PUBLICATION
