- Books Name
- Chemistry Class 10 NCERT based
- Publication
- Grow Career Publication
- Course
- CBSE Class 10
- Subject
- Chemistry
Physical Properties of Organic Compounds
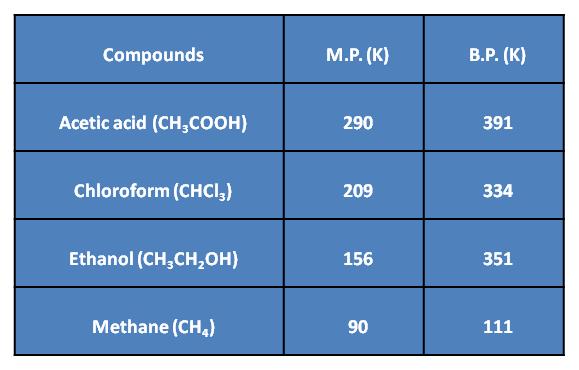
Most of the organic compounds have low boiling and melting point, due to the weak force of attraction (i.e., the inter-molecular force of attraction) between these molecules. Most carbon compounds are poor conductors of electricity, due to the absence of free electrons and free ions.
Chains, Branches and Rings
Saturated and Unsaturated Hydrocarbons
Saturated hydrocarbons: These hydrocarbons have all carbon-carbon single bonds. These are known as alkanes. General formula = CnH2n+2 where n = 1, 2, 3, 4.…..
Unsaturated hydrocarbons: These hydrocarbons have at least one carbon-carbon double or triple bond.
Hydrocarbons with at least one carbon-carbon double bond are called alkenes. General formula = CnH2n where n = 2, 3, 4…..
Hydrocarbons with at least one carbon-carbon triple bond are called alkynes. General formula = CnH2n−2 where n = 2, 3, 4…..
Chains, Rings and Branches
Carbon chains may be in the form of straight chains, branched chains or rings.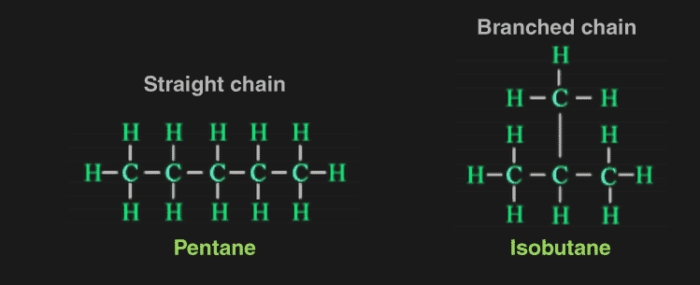
In cyclic compounds,
Structural Isomers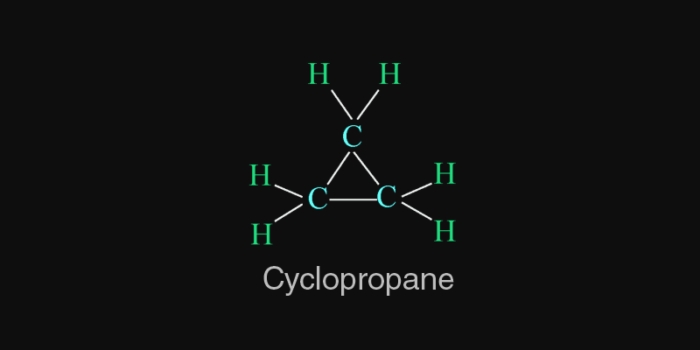
The compounds with the same molecular formula and different physical or chemical properties are known as isomers and the phenomenon is known as isomerism.
The isomers that differ in the structural arrangement of atoms in their molecules are called structural isomers and the phenomenon is known as structural isomerism.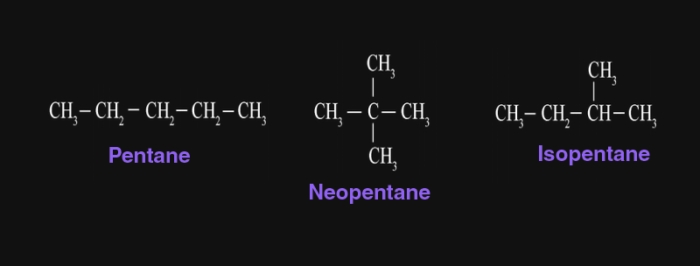
Benzene
Benzene is the simplest organic, aromatic hydrocarbon.
Physical properties: colourless liquid, pungent odour, flammable, volatile.
Structure:
Cyclic in nature with chemical formula, C6H6, i.e., each carbon atom in benzene is arranged in a six-membered ring and is bonded to only one hydrogen atom.
It includes 3-double bonds which are separated by a single bond.
Hence, this arrangement is recognized to have conjugated double bonds and two stable resonance structures exist for the ring.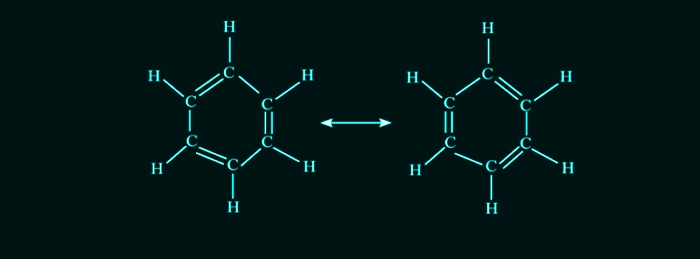
Functional Groups and Nomenclature
Functional Groups
An atom or a group of atoms which when present in a compound gives specific physical and chemical properties to it regardless of the length and nature of the carbon chain is called a functional group.
Classification of Functional Groups
Main Functional Groups:
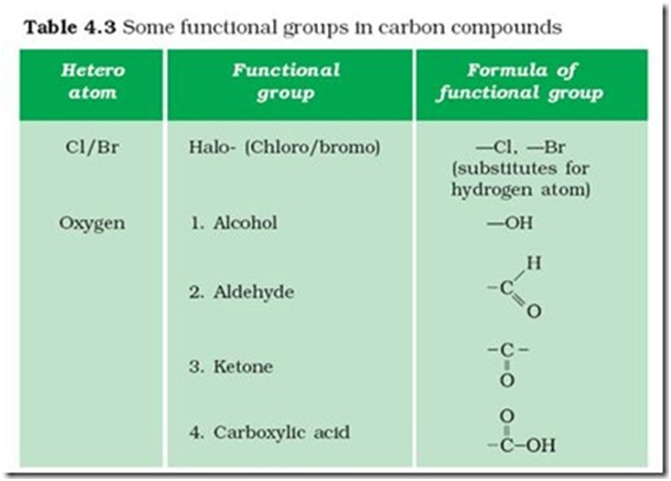
(i) Hydroxyl group (-OH): All organic compounds containing -OH group are known as alcohols. For example, Methanol (CH3OH), Ethanol (CH3−CH2−OH), etc.
(ii) Aldehyde group (-CHO): All organic compounds containing -CHO group are known as aldehydes. For example, Methanal (HCHO), Ethanal (CH3CHO), etc.
(iii) Ketone group (-C=O): All organic compounds containing (-C=O) group flanked by two alkyl groups are known as ketones. For example, Propanone (CH3COCH3), Butanone (CH3COCH2CH3), etc.
(iv) Carboxyl group (-COOH): All organic acids contain a carboxyl group (-COOH). Hence, they are also called carboxylic acids.
For example, Ethanoic acid (CH3COOH), Propanoic acid (CH3CH2COOH), etc.
(v) Halogen group (F, CI, Br, I): The alkanes in which one or more than one hydrogen atom is substituted by- X (F, CI, Br or I) are known as haloalkanes. For example, Chloromethane (CH3Cl), Bromomethane (CH3Br), etc.
Homologous Series
Homologous series constitutes organic compounds with the same general formula, similar chemical characteristics but different physical properties. The adjacent members differ in their molecular formula by −CH2.
Physical Properties
The members of any particular family have almost identical chemical properties due to the same functional group. Their physical properties such as melting point, boiling point, density, etc., show a regular gradation with the increase in the molecular mass.
- Books Name
- Chemistry Book based on NCERT
- Publication
- PRIDE LEARNING PUBLICATION
- Course
- CBSE Class 10
- Subject
- Chemistry
Nature of carbon compounds.
Catenation:- The property of elements to form long chains or rings by self linking of their own atoms through covalentbonds is called catenation. These compounds may have long, branched chains of carbon atoms may be linked by single, double or triple bound. The extent of catenation depends upon the strength of the bounds between the atoms involved in catenation.
Saturated and unsaturated compounds
- Compounds of carbon which are linked by only single bonds between carbon atoms are called saturated compounds.
For example, C - C
Carbon atom linked together with single bound.
But the valencies of each carbon atom remain unsatisfied , so each carbon atom is bonded to 3 hydrogen atoms:-
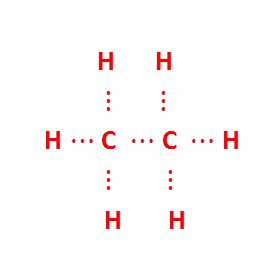
atom is bonded to 3 hydrogen atoms:-
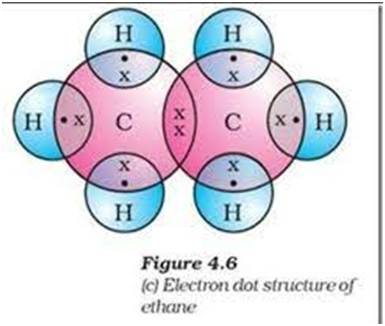
Electron dot structure of ethane
Compounds of carbon having double or triple bounds between their carbon atoms are called unsaturated compounds.
For example,
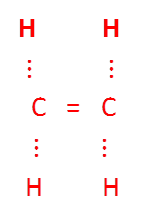
.To satisfy the valency , carbon form double bond.
Strength of compound
The bonds that carbon forms with other elements are very strong, so these compounds because very stable. Carbon form strong bonds is due to its small size. Nucleus hold shaired pair of electrons strougly.
Chains , branches and rings,
Straight chain compounds: the compounds which conatin straight chain of carbon atom e.g butane (
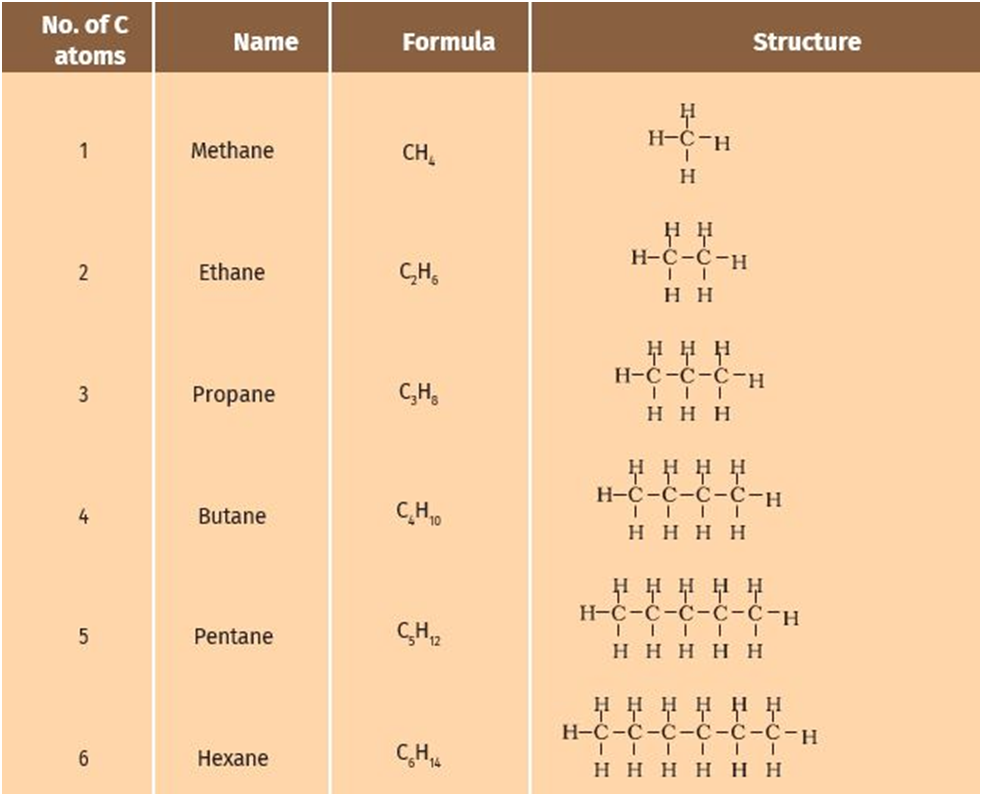
Branched chain compounds: Those compounds which are branched.
E.g. ISO- butane (
Ring compounds:- they are also known as closd chain compounds.
Cyclic compounds are called ring coumpounds.
E.g. cyclohexane (
Cyclopropane (
Hydrocarbons :- All those compounds which contain only carbon and hydrogen are called hydrocarbon. The saturated hydrocarbon which contain single bond are called alkanes. The unsaturated hydro carbons which contain one or more double bonds are called alkanes those containing one or more triple bounds are called alkynes.
Functional group:- The atoms or group of atoms which determine the properties of a copmpond is known as functional group.
E.g. –Cl , -Br ( choro/ bromo alkane
-OH (alcohol)
Homologous series :- A series of compounds in which the same functional group substitutes hydrogen ina carbon chain is called a homologous series. E.g , C
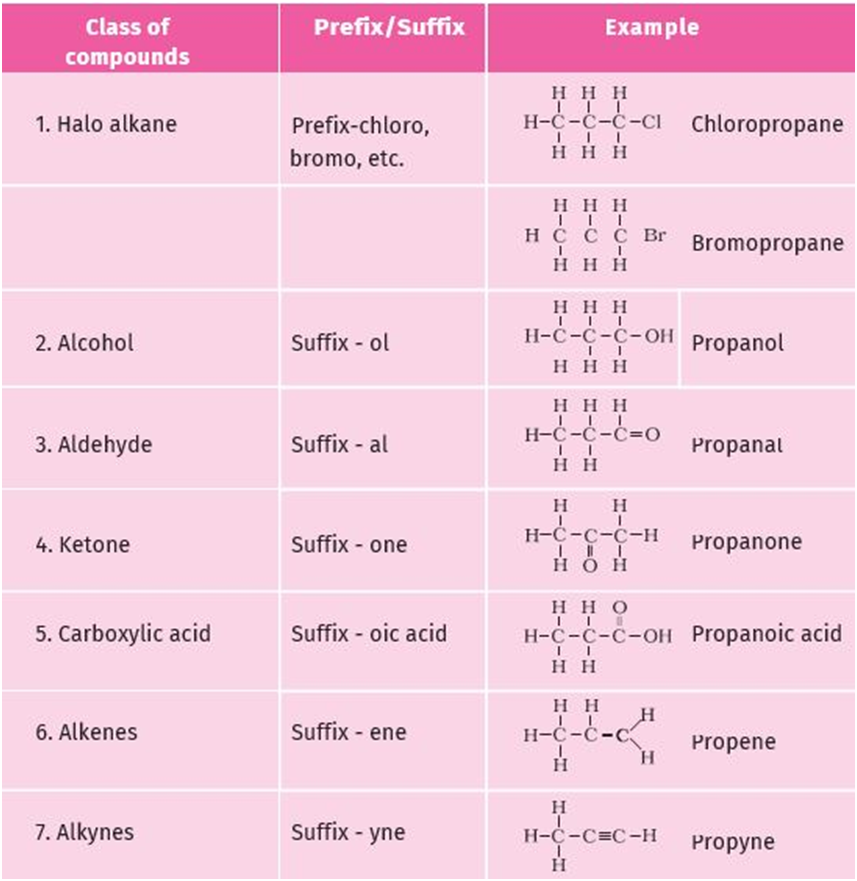
Nomenclature :- For naming organic compounds based on their structures, are followed by UPSC rules.
IUPAC name of an organic compounds consists of 3 parts –
- Prefix :- in case functional group is present , it is indicated in the name of the compound with either as a prefix or as a suffix.
- Word root :- A word root indicates the nature of basic carbon skeleton.
- Suffix :- while adding the suffix to the word rrot, the terminal ‘e’ of carbon chain is removed . if the carbon chain is unsaturated , then final ‘are’ is substituted by ‘en and yne’ respectively for double and triple bonds.

 Grow Career Publication
Grow Career Publication
 PRIDE LEARNING PUBLICATION
PRIDE LEARNING PUBLICATION
