- Books Name
- Chemistry Book based on NCERT
- Publication
- PRIDE LEARNING PUBLICATION
- Course
- CBSE Class 10
- Subject
- Chemistry
TYPE OF CHEMICAL REACTION
There are five type of chemical reactions.
COMBINATION REACTION:- The reaction in which two or more reaction combine to form a single product is called combination reaction They are represented by equation of the following term.
Reactant product
For Example :-
Burning of coal
C(s) +
(carbon) (oxygen) (carbon dioxide)
Formation of water
2
(Hydrogen) (oxygen) (Water)
Formation of slaked line
CaO (s) +
(calcium oxide) (water) (calcium Hydroxide)/ slaked lime
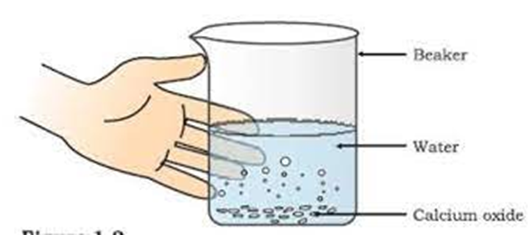
Formation of slaked lime by the reaction of calcium oxide with water.
DECOMPOSITION REACTION :- The reaction I which single compound breaks down to simpler product is called decomposition reaction. They are represented by equation of the following term .
AB
(i) Thermat decomposition :- when decomposition is carried out by heating.
For example
(i) 2 Fe S
(Ferrous sulphate ) (Ferric Oxide)
Green colour Red brown colour
(ii) CaC
(calcium carbonate) ( calcium oxide ) (Carbon dioxide ) Limestone quick lime
(iii) 2Pb (N
(Lead nitrate ) (lead oxide) (Nitrogen dioxide) oxygen
Brown fumes
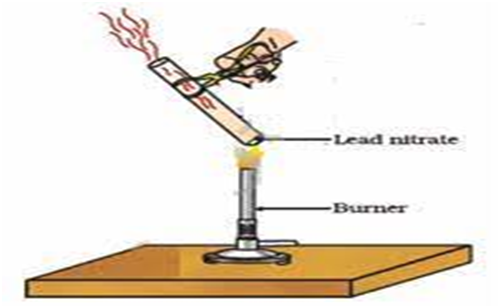
Heating of lead nitrate and emission of nitrogen dioxide
ELECTRONIC DECOMPOSITION :- When decomposition is carried out by passing electricity .
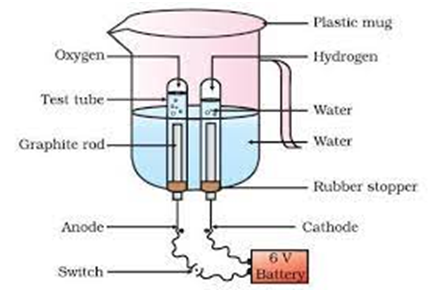
For Example:- (i) Electrolysis of water.
Electric current
2
(Water) (Hydrogen) (Oxygen)
Electrolysis of water is done as follows:-
When decomposition is carried out in presence of sunlight .
For Example:-
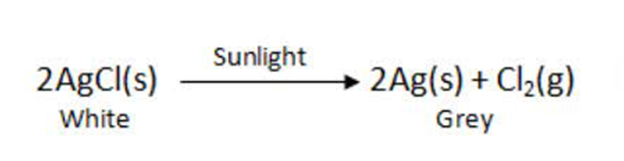
This is why silver chloride turns grey in sunlight because of the decomposition of silver chloride into silvers and chloride by light.
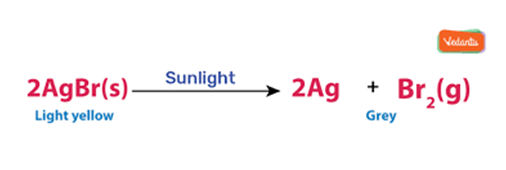
This reaction is used in black and write Photography.
DISPLACEMENT REACTION :- This reaction in which more reactive element displace less reative element from its salt solution is called displacement reaction . they aare represented by equation of the following term.
A + BC
For example :-
(i) Fe (s) + CuS
(Iron ) (copper Sulphate) ( iron Sulphate) ( Copper)
Fe is more reactive than therefore iron (Fe) has displaced copper (Cu) from copper sulphate solution.
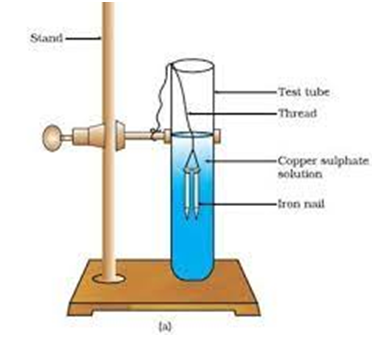
Iron nails dipped in copper sulphate solution.
(ii) Zn(s) + CuS
(Zinc) (copper sulphate) ( Zinc sulphate ) copper
Zinc is more reactive than copper , therefore it displace copper from copper sulphate solution.
(iii) Pb (s) + CuC
(Lead) (copper chloride) ( lead chloride) copper
Lead is more reactive elements than copper, therefore it displaces copper from copper chloride solution.
DOUBLE DISPLACEMENT REACTIVE :- The reaction in which the reactant ions exchange to form new products is called double displacement reaction . they are represented by equation of the following term.
AB + CD
For Example :-
N
(sodium sulphate) (barium chloride) (barium sulphate) (sodium chloride)
White precipitate of BaS
OXIDATION AND REDUCTION :-
OXIDATION
(i) The addition of oxygen to reactant
(ii) the removal of hydrogen from reactant
For Example :-
2 Cu +
(Copper) (oxygen) (copper oxide )
Black substance
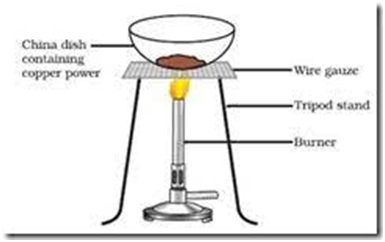
Oxidation of copper to copper oxide
Reduction : - (i) the addition of hydrogen to reactant
(ii) the removal of oxygen from a reactant.
Redox Reactions :- The reaction on written one substance gets oxidisied and other get reduced is known as redox reaction
For example :-
CuO +
In this reaction CuO is reduced to Cu and
ZnO + C
Here, C is oxidized to CO because oxygen is being added and ZnO IS REDUCED TO Zn because O is being removed.
NOTE :
* If a substance loses oxygen during a reactant , it is said to be reduced.
ENDETHERMIC REACTION:- Reaction in which energy is absorded are known as endothermic reaction.
For example :-
CaC
FXOTHERMIC REACTION :- Reaction in which heat is released along with formation of products.
For Example,
C

 Grow Career Publication
Grow Career Publication
 PRIDE LEARNING PUBLICATION
PRIDE LEARNING PUBLICATION
