- Books Name
- Chemistry Class 10 NCERT based
- Publication
- Grow Career Publication
- Course
- CBSE Class 10
- Subject
- Chemistry
Universal indicator
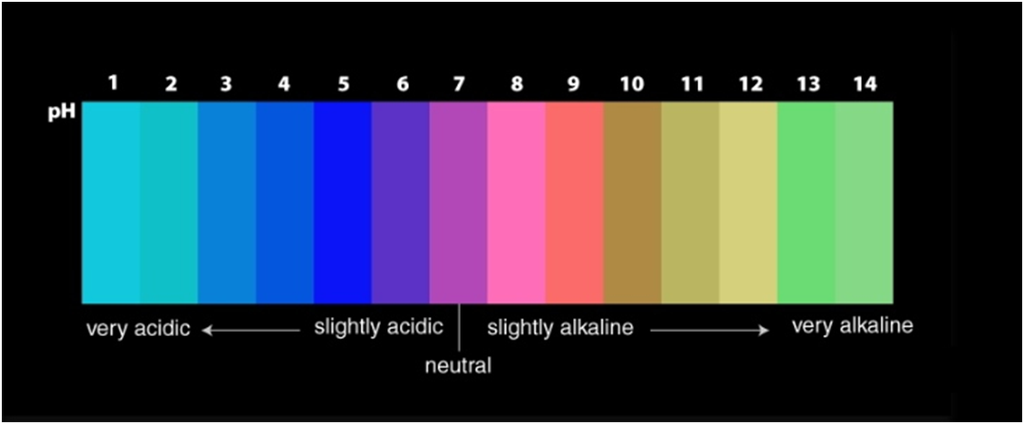
A universal indicator has a pH range from 0 to 14 that indicates the acidity or alkalinity of a solution.
A neutral solution has pH=7
pH
pH=−log10[H+]
In pure water, [H+]=[OH−]=10−7 mol/L. Hence, the pH of pure water is 7.
The pH scale ranges from 0 to 14.
If pH < 7 → acidic solution
If pH > 7→ basic solution
Importance of pH in everyday life
1. pH sensitivity of plants and animals
Plants and animals are sensitive to pH. Crucial life processes such as digestion of food, functions of enzymes and hormones happen at a certain pH value.
2. pH of a soil
The pH of a soil optimal for the growth of plants or crops is 6.5 to 7.0.
3. pH in the digestive system
The process of digestion happens at a specific pH in our stomach which is 1.5 to 4.
The pH of the interaction of enzymes, while food is being digested, is influenced by HCl in our stomach.
4. pH in tooth decay
Tooth decay happens when the teeth are exposed to an acidic environment of pH 5.5 and below.
5. pH of self-defence by animals and plants
Acidic substances are used by animals and plants as a self-defence mechanism. For example, bee and plants like nettle secrete a highly acidic substance for self-defence. These secreted acidic substances have a specific pH.
- Books Name
- Chemistry Book based on NCERT
- Publication
- PRIDE LEARNING PUBLICATION
- Course
- CBSE Class 10
- Subject
- Chemistry
Strongness about Acids or Bases
Strength of acid and base can be determined by universal indicator.
concentrations of
pH scale :- A scale for measuring hydrogen ion concentration in a solution called PH scale.
pH = 7
pH Less than 7
pH more than 7


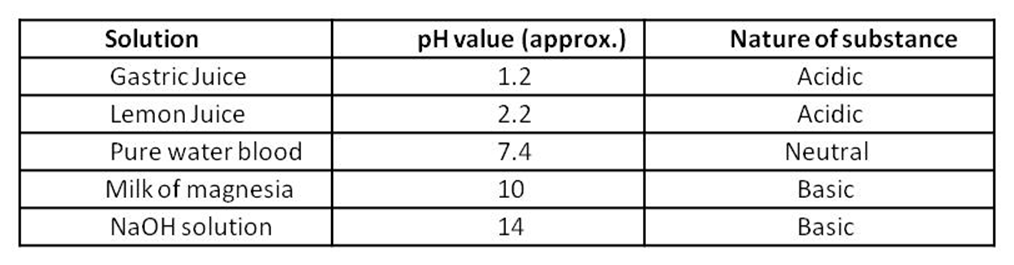
pH of some common substances shown on a pH paper.
Importance of pH in everyday life :-
1 , Plants and animals are Ph sensitive.
- Our body works within the pH range of 7- 7.8 .
- When pH of rain water is less than 5.6, it is called acid rain.
- When acid rain flows into the rivers ,it lower the pH of river water and makes the survival of aquatic life difficult.
2, pH of the soil
- Plants require a specific pH range for their healthy growth.
3, pH in our digestive system.
- Our stomach produce hydrogenchloric acid which helps in digestion without harming the stomach.
- During indigestion, stomach produces more acid and cause pain and irritation.
- To get rid of pain ,prople use mild bases called antacid to neutralize the excess acid. Magnesium hydroxide ( Milk of magnesia) is an antacid.
4, Ph changes as the cause of tooth decay.
- Tooth decay starts when pH of mouth is lower than 5.5
- Tooth enamel is made up of calcium phosphate ( hardest substance in body) .it does not dissolve in water but carodes when pHis low than 5.5
- Using basic toothpaste , tooth decay can be prevented
5, self defence by animals and plants through chemical warfare.
- Bee sting leaves an acid which cause pain and irritation. Bakig soda (mild base) gives relief by rubbing it on stung area.
- Stinging hair of nettle leaves inject methanoic acid causing buring or pain rubbing this with leaf of dock plant give relief.

 Grow Career Publication
Grow Career Publication
 PRIDE LEARNING PUBLICATION
PRIDE LEARNING PUBLICATION
