- Books Name
- ACME SMART COACHING Chemistry Book
- Publication
- ACME SMART PUBLICATION
- Course
- CBSE Class 11
- Subject
- Chemistry
STATES OF MATTER
On the basis of physical properties, matter may be classified into the following three states :
- Solid State
- Liquid State
- Gaseous State
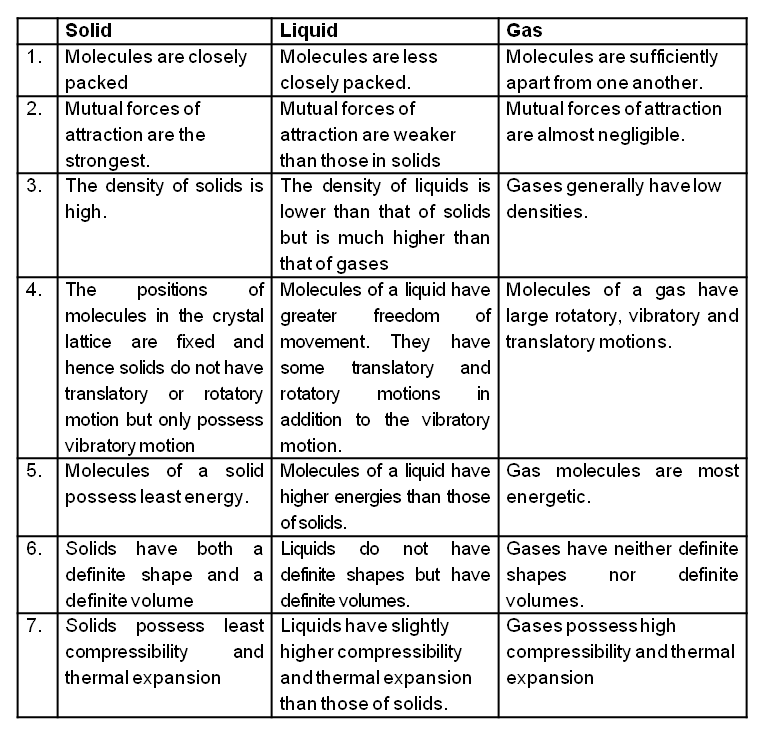
Comparison of the characteristics of a solid, a liquid and a gas
Gases
The molecules of a gas are free to move in all directions. The space in between them is very large. The gaseous molecules possess motion of all three types, namely translational, rotational and vibrational. The molecular forces of attraction between gases are very much weak. Hence a gas has neither a definite shape nor a definite volume.
- Gases are highly compressible
- Gases expand without limits
- Gases exert pressure on the walls of the container uniformly in all directions.
- Gases diffuse rapidly through each other to form a homogeneous mixture.

 Ritan Sheth
Ritan Sheth
