Acids and Bases
- Books Name
- CBSE Class 7 Science Book
- Publication
- Param Publication
- Course
- CBSE Class 7
- Subject
- Science
2.2 ACIDS
The term acid is derived from the Latin word acidus meaning sour. Lemons, oranges and grapes taste sour because they contain citric acid. Tamarind and vinegar contain tartaric acid and acetic acid respectively.
Definition :
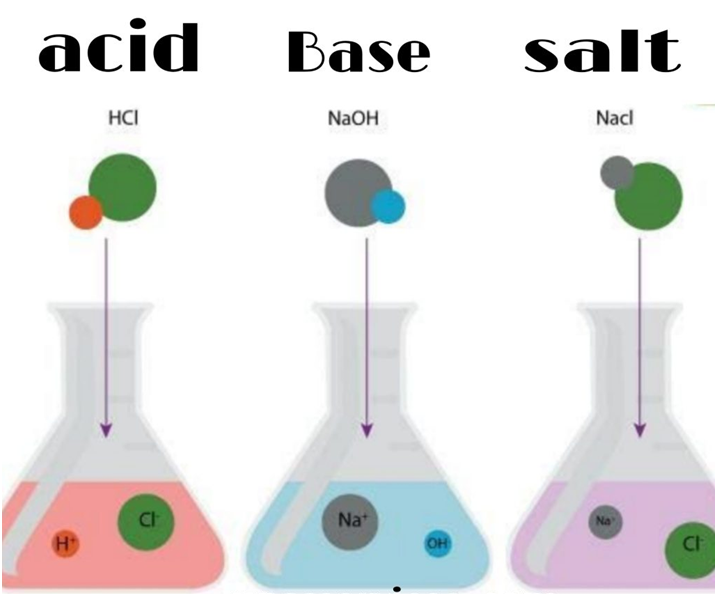
“An acid is defined as a substance which gives H+ ions on dissolution in water.”
e.g.
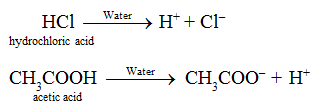
Nitric acid (HNO3), Phosphoric acid (H3PO4) , formic acid (HCOOH) etc. have one and sulphuric acid (H2SO4) has two replaceable hydrogen atom, thus they are acids.
Remember
• Vitamin C which is very important for our body is also an organic acid known as ascorbic acid.
Classification of Acids:
(a) On the basis of occurrence:
(i) Mineral acids : Acids which are obtained from the minerals present in earth’s crust are called mineral acids.
e.g. HCl, H2SO4, HNO3 etc.
(ii) Organic acids : Acids that are found in animals and plants are known as organic acids.
e.g. Lactic acid , citric acid, tartaric acid, acetic acid and formic acid.
(b) On the basis of strength:
(i) Strong Acids :
Acids, which almost completely ionise (break up into ions) in water, are called strong acids.
e.g. Hydrochloric acid (HCl), sulphuric acid (H2SO4), nitric acid (HNO3) etc.
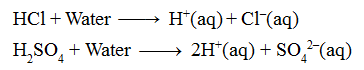
(ii) Weak Acids:
Acids, which partially ionise in water, are called weak acids.
e.g. Carbonic acid (H2CO3), phosphoric acid (H3PO4), formic acid (HCOOH), acetic acid (CH3COOH).
![]()
REMEMBER
• The sharp pain caused by the sting of ants and bees is due to formic acid, which they push into the body or spray on the skin.
• Acids like conc. H2SO4 and conc. HNO3 are corrosive in nature. They destroy organic matter like clothes, paper, wood and cause burn to human skin.
• In general mineral acids are strong while organic acids are weak
(c) On the basis of concentration :
(i) Concentrated acid :
The acid containing very less amount of water is called concentrated acid. HCl is prepared by dissolving HCl gas in water. The solution of this acid is called conc. HCl.
(ii) Dilute acid : The acid containing excess amount of water is called dilute acid. Strength can be decreased by dissolving the acid in more water. In a laboratory, we generally use either concentrated acid or it’s solution diluted to a definite strength.
Advance Learning
Dilution of acids :
It is always desirable to add acid to water, keeping the solution continuously stirred, while preparing dilute solutions of acids, specially mineral acids. We should always slowly add acid to water; otherwise, so much heat is produced during the dilution process that the container, specially that of glass, may break. The hot contents may also cause an explosion and spill on our clothes and body. This may result into serious acid burns.
(d) On the basis of basicity :
(i) Monobasic Acids :
When one molecule of an acid on complete ionisation produces one hydronium ion (H3O+) in aqueous solution, the acid is said to be a monobasic acid.
Examples of Monobasic Acids.
Some examples of monobasic acids are :
(i) Hydrochloric acid (HCl) (ii) Hydrobromic acid (HBr)
(iii) Nitric acid (HNO3) (iv) Acetic acid (CH3COOH)
(v) Formic acid (HCOOH)
(ii) Dibasic Acids :
When one molecule of an acid on complete ionisation produces two hydronium ions (H3O+) in aqueous solution, the acid is said to be a dibasic acid.
Examples of Dibasic Acids :
Some examples of dibasic acids are :
(i) Sulphuric acid (H2SO4) (ii) Sulphurous acid (H2SO3)
(iii) Carbonic acid (H2CO3) (iv) Oxalic acid [(COOH)2]
(v) Hydrofluoric acid (HF)
(iii) Tribasic Acids :
When one molecule of an acid on complete ionisation produces three hydronium ions (H3O+) in aqueous solution, the acid is said to be a tribasic acid.
An example of tribasic acids is Phosphoric acid (H3PO4).
(iv) Tetrabasic Acids :
When one molecule of an acid on complete ionisation produces four hydronium ions (H3O+) in aqueous solution, the acid is said to be a tetrabasic acid.
An example of tetrabasic acids is silicic acid (H4SiO4)
Remember
• The atmosphere of Venus is made up of thick white and yellow clouds of Oil of Vitriol (H2SO4 ).
Acids and Bases
Chapter 5: Acids, Bases and Salts
Many acids and bases occur naturally in nature, such as citric acid in fruits like orange, lemon, etc, tartaric acid in tamarind, malic acid in apples, and lactic acid in milk and milk products, hydrochloric acid in gastric juices.
Similarly, many bases are found such as lime water. We use many of these acids in our day-to-day life, such as vinegar or acetic acid in the kitchen, boric acid for laundry, baking soda for the purpose of cooking, washing soda for cleaning, etc.
Acid and bases
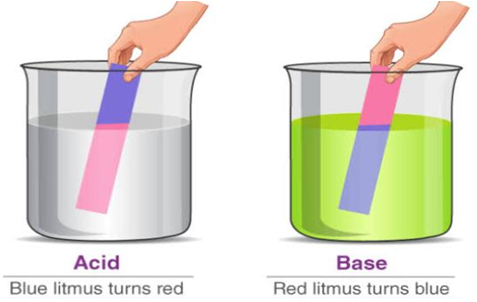
Acids and bases are popular chemicals which interact with each other resulting in the formation of salt and water. The word acid comes from a Latin word ‘acere’ which means ‘sour’.
Natural Indicators Around Us
- Books Name
- CBSE Class 7 Science Book
- Publication
- Param Publication
- Course
- CBSE Class 7
- Subject
- Science
2.4 INDICATORS
It is not possible to taste each and every substance to identify its chemical nature and also, it may be dangerous to touch each and every substance.
To overcome this problem, special types of substances called indicators are used to get to know the chemical nature of substances.
An indicator is a substance which indicates the nature of particular solution whether acidic, basic or neutral. Hence they indicate the change in nature of the solution from acidic to basic and vice versa. Indicators are basically coloured organic substances.
(a) Different types of indicators :
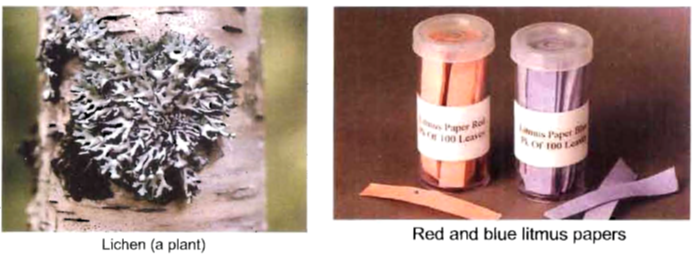
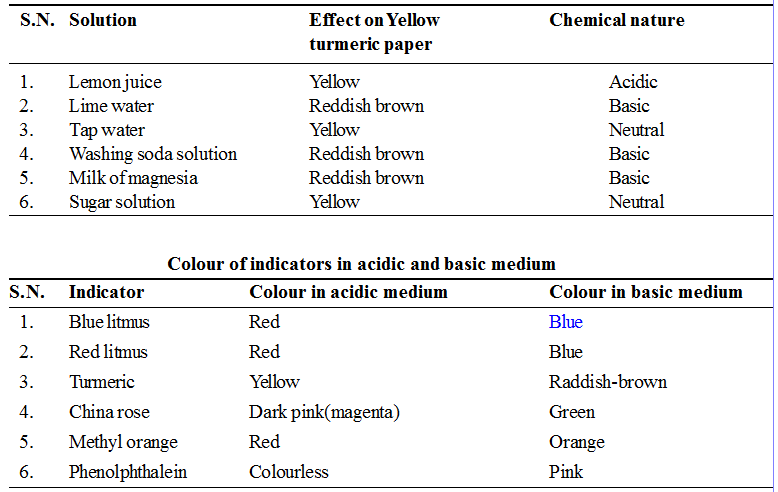
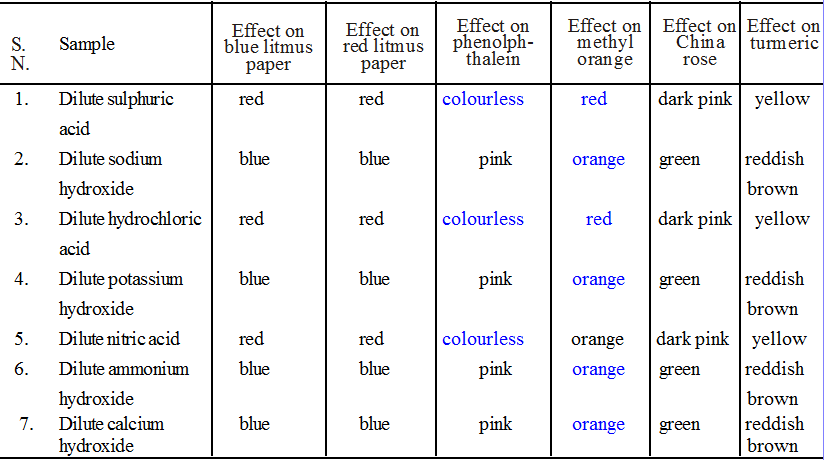
(i) Litmus : Litmus is a purple dye which is extracted from a plant ‘lichen’. It can also be applied on paper in the form of strips and is available as blue and red strips. A blue litmus strip, when dipped in an acid solution acquires red colour. Similarly a red strip when dipped in a base solution becomes blue.
Activity–1
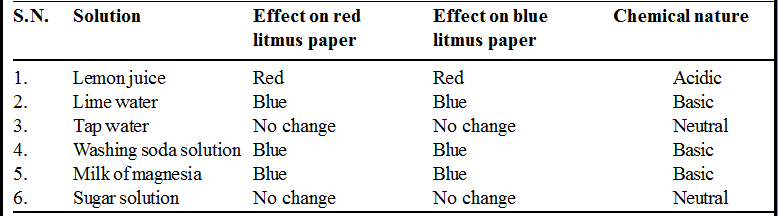
To test the chemical nature of a few substances.
• Collect lemon juice, lime water, tap water, washing soda solution, milk of magnesia, and sugar solution in separate test tubes.
• With the help of dropper, one by one, put a drop of each solution on separate red and blue litmus papers.
• Record your observations in Table.
(ii) Phenolphthalein : It is also an organic dye. In neutral or acidic solution, it remains colourless while in the basic solution, the colour of indicator changes to pink.
(iii) Methyl Orange : Methyl orange is an orange coloured dye and keeps this colour in the neutral or basic medium. In the acidic medium the colour of indicator becomes red.
(iv) Red Cabbage Juice : It is purple in colour in neutral medium and turns red or pink in the acidic medium. In the basic or alkaline medium, its colour changes to green.
(v) Turmeric juice : It is yellow in colour and remains as such in the neutral and acidic medium. In the basic medium its colour becomes reddish or deep brown.
(vi) China Rose : Extract of china rose (Gudhal) petals is of pink colour. It will change into dark pink (magenta) in acidic solution and green in basic solution.
Activity–2
To test the nature of different sbstances using temeric as an indicator.
• Make turmeric paste in a bowl
• Now leave it to dry for 15-20 minutes.
• cut thin strips of yellow turmeric paper
• Collect lemon juice, lime water, tap water, washing soda solution, milk of magnesia and sugar solution in separate test tubes
• With the help of dropper, one by one, put a drop of each solution on the thin strip of yellow turmeric paper.
• Record your observation in Table
Activity–3
To observer the effect of various indicators on acidic and basic solution
• Collect 5 mL each of dilute sulphuric acid, dilute sodium hydroxide, dilute hydrochloric acid, dilute potassium hydroxide, dilute nitric acid, dilute ammonium hydroxide and dilute calcium hydroxide in separate test tubes.
• One by one test the acidic or basic nature of each of the sample solutions with blue litmus paper, red litmus paper, phenolphthalein, methyl orange, China rose and turmeric indicators.
• Record your observation in Table
Natural Indicators Around Us
Natural indicators around us

Some examples of natural indicators are red cabbage, turmeric, grape juice, turnip skin, curry powder, cherries, beetroots, onion, tomato, etc. Some flowers like hydrangeas can determine the acidity or basicity of the soil.
Neutralisation
- Books Name
- CBSE Class 7 Science Book
- Publication
- Param Publication
- Course
- CBSE Class 7
- Subject
- Science
2.5 NEUTRALISATION
The reaction between an acid and a base is known as neutralisation. Salt and water are produced in this process with the evolution of heat. Evolved heat is known as heat of neutralisation .
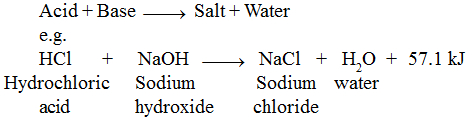
Where 57.1kJ energy is the heat of neutralisation for above reaction. This value remains same if both acid and base are strong. If one out of these is weak then amount of energy released will be lesser than 57.1 kJ
Activity–4
Aim : To observe the neutralization reaction.
Procedure :
• Take a test tube and fill it one-fourth with dilute hydrochloric acid (HCl).
• With the help of a dropper, add 2–3 drops of phenolphthalein indicator (colourless) to it.
• Gently shake the test tube.
• Note down its colour.
You will observe that the solution is colourless. 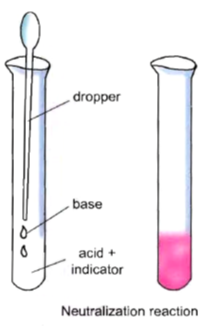
• Now put a drop of dilute sodium hydroxide with the help of a dropper and shake it gently.
• Continue adding dilute sodium hydroxide and shaking it till the pink colour just appears.
• At this point, the solution is just neutral.
• Add a drop of dilute hydrochloric acid to it
• What do you observe now ?
You will notice that the pink colour disappears.
• Again add a drop of dilute sodium hydroxide.
You will notice that the pink colour reappears.
This happens because phenolphthalein is colourless in an acidic medium and pink in a basic medium.
Inference : Drop by drop addition of dilute sodium hydroxide neutralizes dilute hydrochloric acid.
Neutralisation
Neutralization
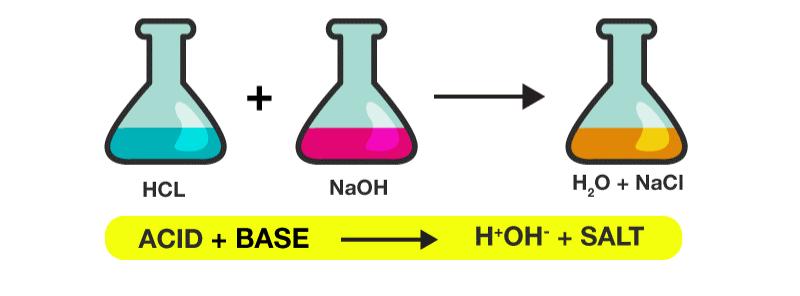
In chemistry, neutralization or neutralization (see spelling differences) is a chemical reaction in which acid and a base react quantitatively with each other. In a reaction in water, neutralization results in there being no excess of hydrogen or hydroxide ions present in the solution. The pH of the neutralized solution depends on the acid strength of the reactants.
Neutralisations in Everyday Life.
- Books Name
- CBSE Class 7 Science Book
- Publication
- Param Publication
- Course
- CBSE Class 7
- Subject
- Science
(a) Neutralisation in everyday life:
(i) Indigestion : People particularly of old age suffer from acidity problems in the stomach which is caused mainly due to release of excessive gastric juices containing HCl. The acidity is neutralised by antacid tablets which contain sodium hydrogen carbonate (baking soda, NaHCO3), magnesium hydroxide (milk of magnesia, Mg(OH)2) etc.
(ii) Ant and bee sting : The stings of bees and ants contain formic acid. Its corrosive and poisonous effect can be neutralised by rubbing soap which contains NaOH (an alkali) or by rubbing baking soda (NaHCO3) or by calamine solution (ZnCO3). The stings of wasps contain an alkali and its poisonous effect can be neutralised by an acid like acetic acid (present in vinegar).
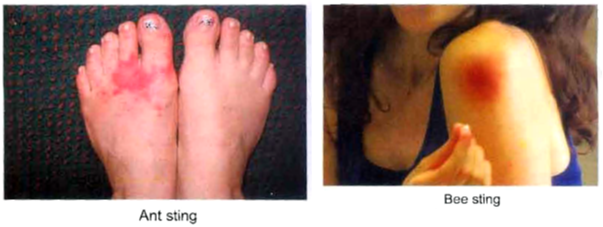
(iii) Soil treatment : Farmers generally neutralize the effect of acidity in the soil caused by acid rain by adding slaked lime (Calcium hydroxide, Ca(OH)2) to the soil.
(iv) Factory wastes : The wastes of many factories contain acids. If they are allowed to flow into the water bodies, the acids will kill fish and other organisms. The factory wastes are, therefore, neutralised by adding basic substances.
POINTS TO REMEMBER
· Atom : The smallest particle of an element that takes part in a chemical reaction is an atom.
· Element : Element is the basic constituent of all matter.
· Chemical Compound : A substance whose each molecule contains two or more atoms of different elements in a fixed ratio is a chemical compound.
· Acid : The substance which contains hydrogen and produces H+ ions in aquesous solution is called Acid. Acids are sour in taste.
· Base: The substance which produces OH– ions in aqueous solution is called the chemical substances which are bitter in taste and soapy base touch.
· Alkalis : Bases which dissolves in water are called alkalis.
· Neutralisation : The reaction between an acid and a base is known as neutralisation.
· Antacid : It is a medicine that neutralize acid formed in the stomach.
· Litmus, turmeric and china rose petal are naturally occurring indicators, while methyl orange and phenolphthalein are prepared in laboratories.
· On the basis of chemical nature, all chemical substances are broadly classified as acidic, basic and neutral substances.
· Acid Rain : When pollutent like sulphur dioxide and nitrogen oxides dissolve in rain water, it forms an acid. The rain of that acid is called acid rain.
Neutralisations in Everyday Life.
Neutralization in everyday life
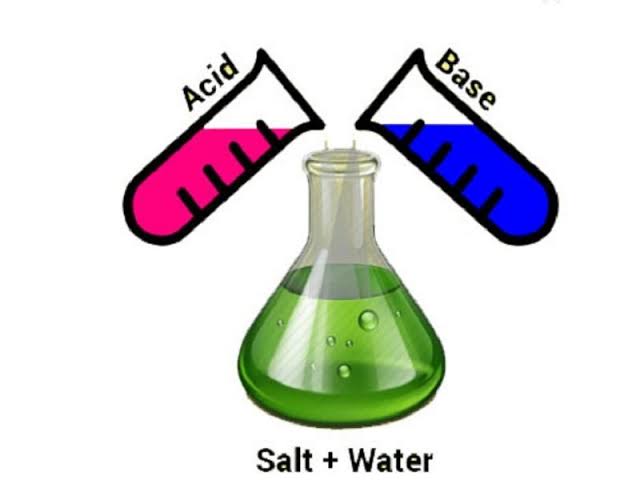
The reaction between an acid and a base is called neutralisation. In everyday life, it is employed in different applications. For example, it is used in the neutralisation of stomach acidity, in the prevention of tooth decay, neutralising the soil, in the treatment of ant’s bites, etc
Acids and Bases Part 2
- Books Name
- CBSE Class 7 Science Book
- Publication
- Param Publication
- Course
- CBSE Class 7
- Subject
- Science
2.3 BASES
These chemical substances are bitter in taste and soapy to touch . The chemical nature of such substances is basic.
Definition :
A base is a compound which gives hydroxyl group (OH–) on dissolution in water are known as bases.
e.g.
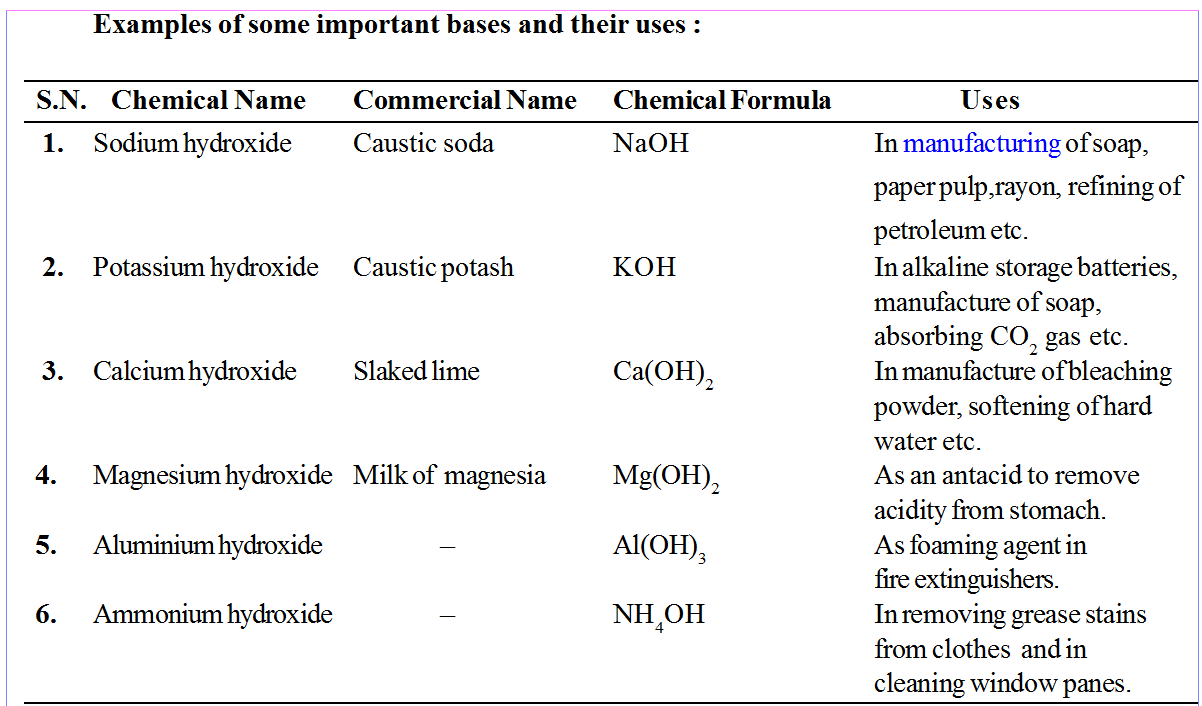
Sodium hydroxide NaOH
Calcium hydroxide Ca(OH)2
Aluminium hydroxide Al(OH)3
Remember
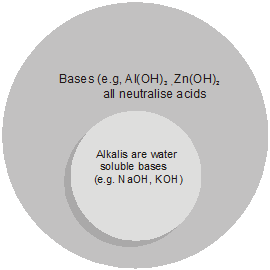
• Alkalis :
Bases which dissolves in water are called alkalis. e.g. KOH, NaOH.
All alkalis are bases but all bases are not alkalis. e.g. Al(OH)3 is a base, but not an alkali.
Remember
• Sodium carbonate (Na2CO3) is commonly called Washing soda.
• Sodium bicarbonate(NaHCO3) is commonly called baking soda.
• CaO is used to neutralize acidic nature of soil.
• Ca(OH)2 is used to prepare mortar, bleaching powder and to neutralize acid in water supplies.
• KOH (caustic potash) is used to conduct electricity between two electrodes.
Advance Learning
• Both NaOH and KOH are deliquescent in nature which means that they absorb moisture from air.
Classification of Bases :
(i) On the basis of strength :
(1) Strong Bases : Bases which are almost completely dissociated in water are known as strong bases.
e.g. Sodium hydroxide (NaOH), potassium hydroxide (KOH), barium hydroxide Ba(OH)2 etc .
![]()
(2) Weak Bases : Bases which dissolve in water only slightly and produce a low concentration of hydroxide ions are called weak bases.
e.g. Ammonium hydroxide (NH4OH), silver hydroxide (AgOH) etc.
(ii) On the Basis of their Concentration :
By the term concentration, we mean the amount of water present in the given sample of alkali solution in water. On the basis of concentration, the alkalis can be classified as under :
(1) Concentrated alkali :
A solution of alkali having a relatively high percentage of alkali in its aqueous solution is known as concentrated alkali.
(2) Dilute alkali :
A solution of alkali having a relatively low percentage of alkali in its aqueous solution is known as a dilute alkali.
If the concentration of alkali in the solution is less than 1 mole per litre, then it is considered to be a dilute alkali.
(iii) On the Basis of their Acidity :
The number of hydroxide (OH–) ions produced by one molecule of an alkali on complete dissociation in water or the number of hydrogen ions (of an acid) with which a molecule of that alkali reacts to produce salt and water only is known as acidity of an alkali.
For water insoluble hydroxides, acidity of the base is equal to the number of OH– ions present in one molecule of that base.
On the basis of acidity, the bases can be classified as under :
(1) Monoacidic Bases (or alkalis) :
When one molecule of the base on complete ionisation produces one hydroxide (OH–) ion in aqueous solution, the base or alkali is said to be monoacidic base.
OR
A monoacidic base (or alkali) may be defined as one whose one molecule reacts with one hydrogen (H+) ion completely to form salt and water as the only products.
Examples of Monoacidic Bases (or alkalis) :
Sodium hydroxide (NaOH), Potassium hydroxide (KOH), Ammonium hydroxide (NH4OH). All these substances produce only one hydroxyl ion on complete ionisation in aqueous solution.
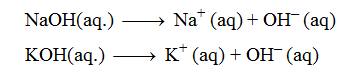
The dissociation of monoacidic bases or alkalis takes place in a single step.
(2) Diacidic Bases (or alkalis) :
When one molecule of a base or alkali on complete ionisation produces two hydroxide (OH–) ions in aqueous solution, the base or alkali is said to be diacidic.
Examples of Diacidic Bases
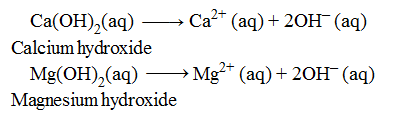
One molecule of both the bases are producing 2OH– ions in aqueous solution, therefore, these are termed as diacidic bases .
(3) Triacidic Bases :
When one molecule of a base or alkali on complete ionisation produces three hydroxide (OH–) ions in aqueous solution, the base or alkali is said to be triacidic base.
Examples of Triacidic Bases :
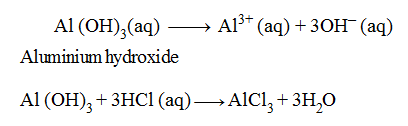
In the above equations, one molecule of Al (OH)3 is producing three OH– ions and one molecule of Al (OH)3 is reacting with three hydrogen (H+) ions to form salt and water only, therefore, it is termed as a triacidic base.

 Param Publication
Param Publication
 Grow Career Publication
Grow Career Publication
