- Books Name
- CBSE Class 7 Science Book
- Publication
- Param Publication
- Course
- CBSE Class 7
- Subject
- Science
INTRODUCTION :
Heat is a form of energy. We know that energy means capacity to do work. So we can say, heat, being a form of energy, can do work. For example, when pressure cooker is heated, steam is formed within the cooker and this steam can lift the lid of the pressure cooker. In this chapter we shall study about the meaning of hot and cold and how things are heated or cooled.
TEMPERATURE - DEGREE OF HOTNESS ORCOLDNESS :
When we touch an ice cube, we say it is cold. Similarly when we touch a cup of tea, we say it is hot. Here we compare the hotness or coldness of an object with that of our hand. But sensing the hotness or coldness by touching is not always true. “The degree of hotness or coldness of a substance is called its temperature.” When two objects at different temperatures are kept in contact, heat flows from hotter object (high temperature) to colder object (low temperature). So direction of heat flow is governed by the temperatures of the objects in contact. It should be kept in mind that exchange of heat continues so long as the temperatures of the two objects remains unequal. Heat flow stops as soon as temperatures become equal which is known as thermal equilibrium.
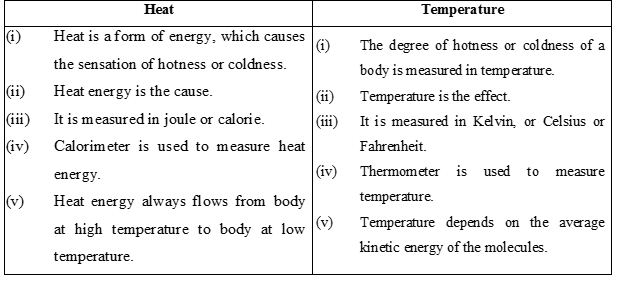
Differences Between Heat and Temperature :
Chapter 4: Heat
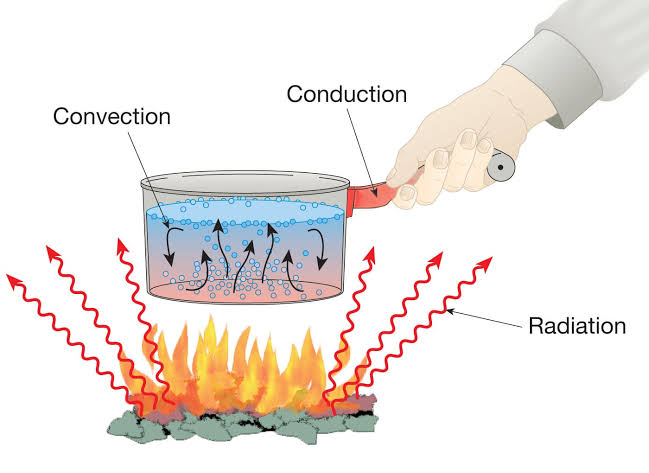
Heat, energy that is transferred from one body to another as the result of a difference in temperature. If two bodies at different temperatures are brought together, energy is transferred—i.e., heat flows—from the hotter body to the colder. The effect of this transfer of energy usually, but not always, is an increase in the temperature of the colder body and a decrease in the temperature of the hotter body.
Hot and cold
In our daily routine, we come across a number of objects, out of which some are hot while other objects are cold, e.g. when a frying pan kept on a burning gas stove becomes hot but the handle of the pan is cold. Even among the hot objects, some objects may be hotter than the other.

 Param Publication
Param Publication
 Grow Career Publication
Grow Career Publication
